Basic Knowledge of Tungsten
- Details
- Category: Tungsten Information
- Published on Monday, 15 July 2013 10:45
- Hits: 2210

Tungsten is silvery white metal, melting point is as high as 3400℃; High hardness, high density, high temperature strength. Tungsten are mainly used for the production of iron and tungsten carbide. Tungsten, molybdenum, cobalt and chromium and other components of the heat resistant alloy used to make tools, metal surface hardening materials, with blade drawing machine. With tungsten, molybdenum, tantalum, niobium fusion of gold to. Tungsten copper and silver tungsten alloy used to make the light bulb, the tube parts and electric arc welding electrode. Some of the compounds can be tungsten fluorescent agents, pigment, dye, etc.
Tungsten is widely used in petroleum and natural gas, mining, electronic, metal processing, machinery and equipment, heavy manufacturing, the department that the application of tungsten to 85% of the total, other applications in military, nuclear energy and aerospace industry, etc. With the development of economy, the progress of science and technology of China's tungsten, application scope is gradually expand, to increase, and great varieties of products to meet the national economic construction and the needs of the construction of national defence and military affairs.

Tungsten Manufacturer & Supplier: Chinatungsten Online - http://www.chinatungsten.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten Picture Center: http://picture.chinatungsten.com
Tungsten Video Center: http://v.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
Tungsten Alloy for Die Casting
- Details
- Category: Tungsten Information
- Published on Monday, 15 July 2013 10:35
- Hits: 2082
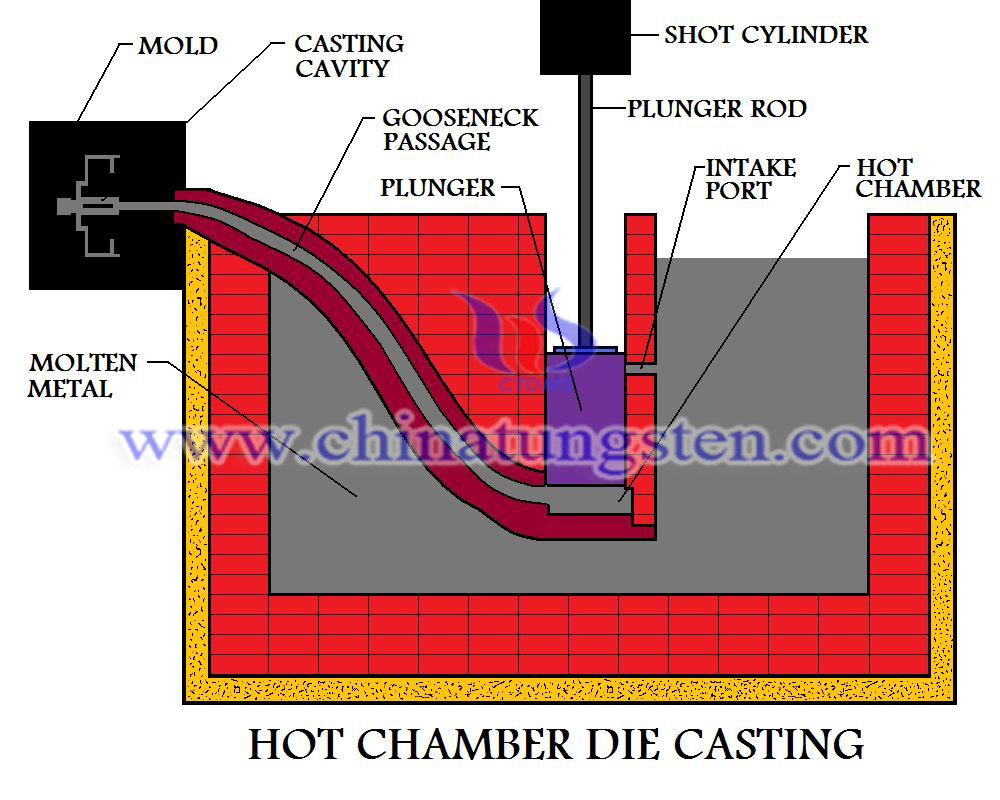
Hard steel production is usually suppressed by a solid metal hole, known as the mold, in such a high-intensity pressure, as it is squeezed out like noodles. Can you imagine how much this incredible hole kind of power? In some cases, will use the diamond or sapphire (similar hardness) die, but die casting, made of tungsten alloy is still very high intensity, even at very high temperature.
Because of its heat resistance, high hardness, high toughness, tungsten alloy is very suitable for production of die casting, components, etc., particularly to suppress copper alloy, aluminum, zinc, brass. Die casting is made of tungsten alloy, high melting point, not oxygen (except in the case of impure).
As the tungsten alloy is very strong, which also ensures it a longer life than the traditional mold, but also to ensure minimum shrinkage porosity. Therefore made of tungsten raw materials such as the die casting to ensure product quality and high standards

Tungsten Manufacturer & Supplier: Chinatungsten Online - http://www.chinatungsten.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten Picture Center: http://picture.chinatungsten.com
Tungsten Video Center: http://v.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
Proper Usage of Tungsten alloy
- Details
- Category: Tungsten Information
- Published on Monday, 15 July 2013 10:16
- Hits: 2254
70% of tungsten alloy in the world is made in China, and 70% of tungsten alloy in China is made in the middle,Hunan and Jiangxi Province. Jiangxi is the center of raw materials, and Hunan is the processing center of tungsten alloy,tungsten copper and related products such as tungsten sheet, tungsten ball,tungsten wire,tungsten rod and so on. All of them are widely used for many industries ,machinery, electronics, chemical, air, miliary, medical, il. They are well known in the world with good quality, competitive price and excellent service.
At present, The demand of tungsten alloy and tungsten copper is getting greater and greater becasue of economy developing speedly. But The total of tungsten alloy and tungsten copper in the World, in China are limited. We need to save the limited resources and recommend international advanced science and technology to exploit tungsten products. At the same time, we must note the environmental protection.
Tungsten Manufacturer & Supplier: Chinatungsten Online - http://www.chinatungsten.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten Picture Center: http://picture.chinatungsten.com
Tungsten Video Center: http://v.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
What Are the Different Types of Radiation Shielding?
- Details
- Category: Tungsten Information
- Published on Monday, 15 July 2013 09:47
- Hits: 2118

Radiation shielding is the use of tungsten alloy to protect against ionizing radiation. Radiation occurs when energy is emitted from one substance and travels out in straight waves, possibly penetrating another substance. When this energy is absorbed, it can have the effect of exciting or destabilizing atoms. If a certain radiation penetrates an animal, it can have harmful impacts on the body, sometimes causing cancer or deformities. Shielding uses specific types of material, such as a leaded glass pane, a lead apron, or packed dirt, to act as a barrier between the body and the source of radiation.
However, compared with other materials, tungsten alloy is the most suitable for radiation protection.As tungsten alloy is the right material for radiation protection, as its combination of radiographic density (more than 60% denser than lead), machinability, good corrosion resistance, high radiation absorption (superior to lead and steel), simplified life cycle and high strength.

Tungsten Manufacturer & Supplier: Chinatungsten Online - http://www.chinatungsten.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten Picture Center: http://picture.chinatungsten.com
Tungsten Video Center: http://v.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
Tungsten Alloy Shielding for Plasma Accelerator
- Details
- Category: Tungsten Information
- Published on Monday, 15 July 2013 09:38
- Hits: 2096

Tungsten alloy shielding is necessary for plasma accelerator. Primary radiation is accelerated electron. Generally, electron beam which thickness is 1~2mm and shoots to target is focused and energy is also centralized. Primary radiation cannot damage to health when it locates at vacuum of accelerator. But as electron beam which is external application, it may damage to staffs for its high radiation strength.
Bremsstrahlung, caused by high energy electron beam bombarding to target interacts with X-ray, which energy is higher than 10MeV and accelerator parts. The process is called photon-neutron reaction and neutron caused during reaction is secondary radiation. Otherwise, some parts of accelerator may produce X-ray even most of its systems are not working.
Tungsten alloy shielding for plasma accelerator is used to protect staffs from radiation. Depending on heaviest density but small capacity, tungsten alloy material is more and more popular be used for making tungsten alloy shielding to protect body from plasma accelerator radiation. Compared with lead, tungsten alloy is much smaller but with heavier density, which is very helpful for high radiation absorption. It is more than 60% denser than lead, meanwhile, it has excellent machinability, good corrosion resistance. The most important thing is that, tungsten alloy is environment-friendly.

Tungsten Manufacturer & Supplier: Chinatungsten Online - http://www.chinatungsten.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten Picture Center: http://picture.chinatungsten.com
Tungsten Video Center: http://v.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
Where Tungsten is found
- Details
- Category: Tungsten Information
- Published on Friday, 12 July 2013 11:16
- Hits: 2216

Tungsten makes up a very small portion of the Earth’s crust and is a very scarce metal. Tungsten makes up just a miniscule 1.1 parts per million of the Earth’s crust. Just to get an idea, other elements such as Iron (about 63,000 parts per million) and lead (about 10 parts per million) make up a larger portion of the Earth’s crust. Tungsten’s closest “relatives” in the periodic table are usually considered to be molybdenum and tin, which make up similar parts per million of the Earth’s crust: 1.1. and 2.2 respectively.
Although, it sounds very small in the grand scheme of the parts per million of the Earth’s crust, the Earth’s crust is enormous and therefore there is still plenty of tungsten. However, it is never naturally found as a metal. Tungsten can only be found as a mineral, meaning it is found combined with other elements to form compounds. In fact, there are more than twenty tungsten minerals that can be found in nature, but only two of those are used to extract metal: scheelite and tungsten.
Tungsten is usually associated with a tin ore known as cassiterite that is usually found near granitic rocks. Tungsten is black-brown mineral and is one of the main sources for tungsten, as mentioned above. When found in its natural state, wolframite will contain varying levels of manganese tungstate and also iron tungstate. Depending on the composition of tungsten mineral, it will be called a different name. If the mineral contains more than 80% manganese tunsgstate is known as hubnerite, whereas if the mineral consists of more than 80% iron tungstate it will be known as ferberite.
Whereas tungsten will be found in that black brown color, Scheelite varies in color from white to green. Also, under ultraviolet light, pure scheelite will glow. Unlike wolframite that will have manganese tungstate or iron tungstate, scheelite will consist of calcium tungstate. Whether it is found as tungsten or scheelite, the tungsten metal can eventually be turned into a variety of things, including the tungsten carbide rings that are becoming increasingly popular.

Tungsten Manufacturer & Supplier: Chinatungsten Online - http://www.chinatungsten.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten Picture Center: http://picture.chinatungsten.com
Tungsten Video Center: http://v.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
Tungsten Alloy Cube for Clock
- Details
- Category: Tungsten Information
- Published on Friday, 12 July 2013 10:56
- Hits: 2069

When clock vibrates, there is an eccentric motion, which is caused by eccentric motor with vibrating components occurs. As the center of the gravity is eccentric, and is not in the rotor of motor, then the clock vibrates. In this case, machinery component with good properties of wear resistant and high specific gravity is required. Tungsten alloy cube is the best material to make tungsten alloy vibrator used in clock.
Tungsten alloy cube is excellent material for making tungsten alloy vibrator. Since the density of tungsten alloy cube is so high and the maximum density should be 18.6g/cm3. Tungsten alloy cube is popular where small component with relatively large mass is needed, for example: the tungsten alloy vibrator used in clock.
Tungsten alloy vibrators in clock are one of our leading products. Compared with other materials, tungsten alloy has the advantages of accurate weight, and non-magnetism. In particular, since a motorized weight usually produces the tungsten alloy vibrators, lighter-weight phones may have weaker vibrating mechanisms.

Tungsten Manufacturer & Supplier: Chinatungsten Online - http://www.chinatungsten.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten Picture Center: http://picture.chinatungsten.com
Tungsten Video Center: http://v.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
Tungsten Paperweight
- Details
- Category: Tungsten Information
- Published on Friday, 12 July 2013 10:27
- Hits: 2397

Paperweights are decorative objects, designed to hold sheets of paper on a surface to prevent wind from blowing them away. Tungsten paperweights have a long history in China. It can be traced back to the birth of paper.
In ancient China, tungsten paperweights were necessary equipment in sanctum. For one of the reason was Chinese paint art and handwriting always use brush and larger paper. Popular size of paper may 300mm x 500mm, and the large size paper may be larger than 2000mm x 1000mm. Tungsten paperweights are now important addition to "wen fang si bao" (the four treasures of the study, i.e. writing brush, ink stick, ink slab, paper), were very important for everyone who can read and write.
In the West, first documented appearance of paperweight can be traced to the Exhibition of Austrian Industry held in Vienna in 1845. Tungsten paperweights of Pietro Bigaglia of Venice were displayed at this exhibition. Knowledge of their existence was reportedly soon brought to the attention of the Saint-Louis glass factory in France.
Tungsten paperweights are composed of metal alloy, tungsten heavy alloy. The alloy allows for maximum hardness and rigidity without sacrificing tensile strength. Tungsten paperweights are polished with diamond tools, and it takes a brilliant high polish and resists scratching longer than any metal ever offered to the public. Roughly ten times harder than 18k gold and four times harder than titanium, tungsten paperweights will never bend or lose their shape. Paperweight made of tungsten can be last for a long time.
It is also possible to have "custom" tungsten paperweights made to your specifications. Tungsten Paperweights never worn, never rust, high-density, high-performance paperweight!

Tungsten Manufacturer & Supplier: Chinatungsten Online - http://www.chinatungsten.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten Picture Center: http://picture.chinatungsten.com
Tungsten Video Center: http://v.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
Tungsten Heavy Alloy Turbo Engine
- Details
- Category: Tungsten Information
- Published on Friday, 12 July 2013 10:15
- Hits: 2342

Tungsten heavy alloy turbo engine is the heat engine which is conditioned by its maximum intake temperature, and turbo engines are limited by the behavior of the constituent materials of the articles that are most exposed to heat and constraints.
Concerns for environmental protection have led designers of aviation tungsten heavy alloy turbo engine to search for means to reduce the proportion of pollutants in the exhaust gases of the engines. It is known that the principal problems in the matter of pollution of aviation tungsten alloy turbo engines are, on the one hand, the emission of carbon monoxide, of hydrocarbons, and of various unburnt residues during operation on the ground and, on the other hand, the emission of nitrogen oxides and of particles during take-off and during cruising at altitude. Therefore, tungsten alloy turbo engines are increasingly accepted by public.
Tungsten heavy alloy turbo engine is generally of optimized rating for take-off or near take-off operation. This signifies that, in the primary zone of the combustion chamber, a fraction of the air flow of the compressor is introduced so that, with the injected fuel, the fuel-air mixture in this zone would be essentially stoichiometric in turbo engines. Under these conditions, due to the levels of temperature and high pressures, as complete as possible a combustion is obtained, combustion yields greater than 0.99 are attained, the speeds of the chemical reaction being optimum for these stoichimoetric mixtures.
The first two times can be considered negligible at high ratings because of the pressures which are attained, but it is not so at low ratings. In fact, in order to increase the speed of the vaporization of the fuel, it must be transformed into fine droplets, which, in normal operation, is easily realized by the conventional mechanical atomizing injector, but the performance which is obtained in the lower ratings is poor. This is due to the fact that, if the fuel is well divided into droplets, these are poorly mixed with air in the primary zone and local zones would appear which have a richness which is too high. In the end, it would be necessary that each droplet would have around it the quantity of gas necessary for its vaporization and for its combustion, i.e., a quantity of gas which results in a stoichiometric mixture with the oxygen molecules after complete vaporization. In order to accomplish this, systems such as aerodynamic injection have been proposed. Aerodynamic type injectors generally comprise whirling, or swirled vanes through which the air from the compressor is introduced, which serves to atomize the fuel. An air/fuel pre-mixture is thus obtained. All of these solutions, which allow an improvement in the combustion yield have, however, a maximum efficiency only for values sufficient for the pressures and temperatures of the air at the chamber inlet. All of these factors are advantageous for a reduction of the reaction times and could lead to a reduction of the length of the combustion chamber for tungsten heavy alloy turbo engine and thus to a limitation of the dwell time of the gases in the latter. In the whole working environment, material of heat-resistance is required, therefore, tungsten heavy alloy for turbo engine is widely used for that.
A first objective of tungsten heavy alloy turbo engine is to provide a novel solution to the problem of low operating combustion for a chamber which includes aerodynamic type or pre-atomization injectors, which are mounted in the base of the chamber. In fact, in the case of a conventional chamber of tungsten heavy alloy turbo engine, which is arranged to provide a stoichiometric mixture at take-off, about one-third of the air flow necessary for the combustion of tungsten alloy turbo engine is introduced in the injection system and two-thirds by the primary orifices.

Tungsten Manufacturer & Supplier: Chinatungsten Online - http://www.chinatungsten.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten Picture Center: http://picture.chinatungsten.com
Tungsten Video Center: http://v.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
How Tungsten is Isolated and Refined
- Details
- Category: Tungsten Information
- Published on Friday, 12 July 2013 09:35
- Hits: 2179

Once the tungsten ore that was extracted from the earth is ready for processing and has been prepared. The process in preparing the tungsten is the same as has been used since it was initially extracted by the Spanish brothers and chemists Juan Jose and Fausto d’Elhuyar y de Suvisa. However, unlike the method used by the d’Elhuvar y de Suvisa brothers, modern extraction and preparation requires an extra, complicated step. Today, a complex chemical extraction is produced during the process. The chemical is called ammonium paratungstate, or more commonly known as APT.
APT is produced in two different ways. The methods are acid leaching and autoclave-soda. Ore is mixed with sodium carbonate under high temperatures and pressure in the autoclave-soda process. APT crystals are formed after ammonia is added to sodium tungstate solution.
In the acid leaching process, tungsten ore is broken down using hydrochloric acid making solid tungstic acid and calcium chloride. Ammonia is used as in the autoclave-soda process. Ammonia is used to dissolve tungstic acid. After evaporating and filtering the mixture, crystals of APT are produced.
Now, you may be wondering what crystals have to do with the tungsten carbide rings you purchased at your local or online retailer. Well, the process is still yet to be completed. The next part of the process is to make tungsten oxide.

Tungsten Manufacturer & Supplier: Chinatungsten Online - http://www.chinatungsten.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten Picture Center: http://picture.chinatungsten.com
Tungsten Video Center: http://v.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com









 sales@chinatungsten.com
sales@chinatungsten.com