Alcohol and Tungsten Bronze Powder Photochromic Effect
- Details
- Category: Tungsten Information
- Published on Wednesday, 04 May 2016 17:42
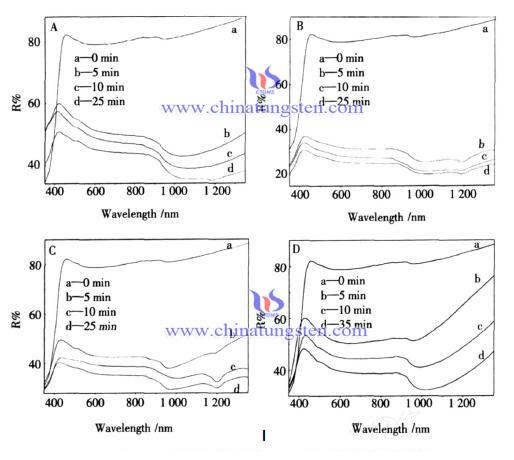
Sodium tungstate as raw material, prepared bronze tungsten oxide powder by hydrothermal synthesis method. The color of the powder turns into blue from light blue after 310 nm ultraviolet light irradiation, the color of the film gets deeper gradually with the increasing exposure time. Figure 1 shows the reflection spectrum of the powder of which the surface adsorbing ethanol, propanol and butanol after the illumination of different times. Compared with photochromic properties of powder without absorbing alcohol, with the extension of irradiation time, the reflected intensity of curve gradually decreased, especially the changes of the absorption peaks around 1 050 nm are obvious.
But after the powder adsorbs different kinds of alcohols, the absorption peak shapes have changed, the reflecting intensity at high wavelength is greatly reduced, and significantly reaching saturation and absorption take less time than when there is no absorption of alcohol, generally reducing 10 min. This may due to the reducing properties of alcohol, so that the powder produced more W5+ ions. In the three alcohol adsorption environment, propanol adsorbed powder is the best in color changing, the second is butanol adsorbed powder, the ethanol adsorbed is poor, the reason of that may be propyl of alcohol has a strong ability to push electrons, so that the hydrogen is easy to be away on the alcohol, and the carbon chain is relatively short, will not hinder the contact of propanol O - H and the tungsten oxide surface.
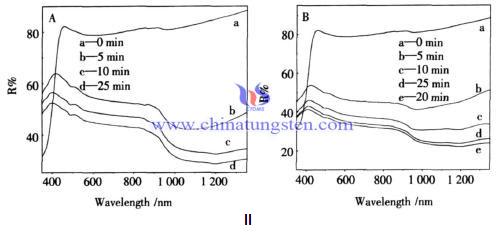
Figure 2 is the reflection spectrum of powder surface adsorbed ethylene glycol and glycerol respectively after the illumination of different times. As can be seen, which is similar with the photochromic curve of the powder in monohydric alcohols environment, with the extension of irradiation time, the reflection intensity of the curve decreases. Compared with monohydric alcohols, phototropy of powder adsorbed glycerol photochromic, with the alcohol metadata increases, the time to reach the required saturable absorption is shorter, and reflection intensity further decreases, it may be that the metadata increasing provides more alcohol O - H to contact with the tungsten oxide surface, thereby generating more W5+.


| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |