Franz Magnetic Separator and Sodium Polytungstate Solution Separating Pure Minerals
- Details
- Category: Tungsten Information
- Published on Friday, 15 January 2016 18:53
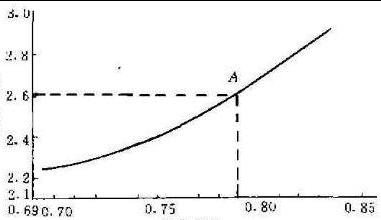
Polytungstate Solution is a kind of avirulent insipidity, neutral, stable and new heavy liquid. It is suitable for the separation of vast majority of oxides and oxysalts. It has been widely applied in the separation experiment of fine mineral and conodont. Choose relative density of heavy liquid formula according to the formula figure before using, for example, to separate microcline and apatite, the relative density of 2.6 heavy liquid can be produced under 20 ℃, namely point A of figure.
This paper introduces the example of Franz magnetic separator and sodium polytungstate solution separating pure minerals. Sample is olive leucite melilitite and experimental steps are as follows:
1. Use anvil to crush and sieve the rocks hand specimen on the oil press. Choose 0.075mm to 0.250mm particles to wash and dry. Remove magnetite by hand magnet.
2. Respectively choose side angle of 20°, 5 A, 1.2 A current and 5° side angle, 1.0 A current to conduct magnetic separation. The final separation product in the non-magnetic end is rich in calcite, apatite, leucite, six-party potassium nepheline.
3. Dissolve 168 g sodium polytungstate powder in 32 mL distilled water fully. Prepare the relative density 2.94 heavy liquid under room temperature to put in heavy funnel. Pour the ultimate separation product in a funnel.
4. Apatite, because of the large relative density rapid subsidence in the bottom of the funnel, let stand for 15 min after turning the funnel below switch, release fallout, with suction filter for heavy liquid will apatite pure sample after repeated washing to dry. Then release all heavy liquid in the funnel and the suction filter collection.
5. Look the microscopic examination, after the operation the apatite is close to 100% purity can be directly used in X-ray powder diffraction and Raman spectra of the test.
Above all, the combined method of Franz magnetic separator and sodium polytungstate solution because of convenience, feasibility, non-toxic and efficiency has become common method of pure minerals separation in laboratory. The wide application of this method will greatly reduce monotonous labor of mineral researchers selecting single mineral microscopically.
| Sodium Tungstate Supplier: Chinatungsten sodium-tungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |