Behavior of Tin in Producing Ammonium Paratungstate 1/3
- Details
- Category: Tungsten Information
- Published on Tuesday, 05 January 2016 19:48
In the production of ammonium paratungstate (APT) and tungsten smelting process, tin is extremely harmful in many impurities, and difficult to remove completely. Even a small amount tin can cause deadly in mechanical and physical properties, the necessity of removal is self-evident. According to GB/T10116-1988, mass fraction of tin in grade 0 APT should be less than 1 * 10-6, and in grade 1 it supposed no more than 3 * 10-6. With the reducing of tungsten concentrate, the mass fraction of impurities such as tin becoming higher and higher, and that makes APT production manufacturers great difficulty to keep quality stable. So it becomes very important to understand the behavior of tin in metallurgy.
Due to the complicated geological, different forms and proportion of tin exist in different tungsten ores. Different leaching rate is shown as below:
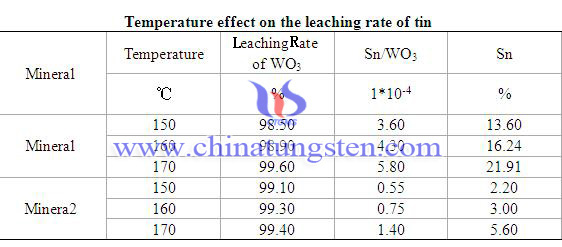

The most important minerals in tungsten metallurgical are tungsten scheelite and tungsten concentrate, cassiterite (SnO2) and tetrahedrite tin (Cu2FeSnS4) exist with them. Tungsten scheelite couldn't conduct electric, but SnO2 could. Tungsten concentrate has weak magnetism, however SnO2 hasn’t. SnO2 couldn't dissolve in acid and alkaline solutions, but, could react with molten sodium hydroxide and create sodium stannate which could soluble in water. Acid decomposition method is mainly used for tungsten scheelite. Usually use hydrochloric acid of density among 1.14~1.15, control temperature in 105-107℃. In the reaction process, tin exists in the state of cation, and gone with filtrate. What is worth to mention is that SnO2 don’t react with acids and leave in coarse tungstate. Then in the process coarse tungstate dissolves in ammonia, SnO2 isn’t reacting with ammonia, thus remained in Solid slag to separate with tungsten.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com