Ammonium Paratungstate Applied in CO2 Conversion Into Methanol
- Details
- Category: Tungsten Information
- Published on Sunday, 30 May 2021 21:13
With the increasing demands of energy brought by traditional fossil fuels, substitutes for clean and sustainable energy sources has gained a lot research interest. Meanwhile, the enormous amount of greenhouse gases such as carbon dioxide (CO2) emitted into in the atmosphere had caused global warming and climate change. One of the strategies that have been adopted to regulate CO2 in atmosphere is carbon capture and sequestration (CCS), but this kind of isolation of CO2 is commercially not viable due to the high cost involved in the process and also the difficulties involved in the long-term storage of CO2. Another strategy for mitigating CO2 emission is to develop a reliable technology to convert CO2 into low carbon fuels such as methanol, methane, carbon monoxide, and formic acid.

Tungsten oxide is a photo-catalyst well known for many photo catalytic applications, is found to be completely inactive in the photo catalytic process of converting carbon dioxide into methanol. Thus, WO3/In2O3 nano photocatalyst had been developed to applied in conversion of CO2 into methanol, the photo catalytic reaction with this composite was successful and resulted in a methanol yield of 496 μmol g−1 h−1.
The preparation method of WO3/In2O3 photocatalyst is as below:
Indium nitrate hydrate, In(NO3)3·xH2O (99.99%), Ammonium paratungstate ((NH4)10W12O415H2O) and SBA-15 were procured from Sinopharm Chemical Reagent Company (China) and the deionized water used in this experiment has a resistivity of about 18.00 MΩcm. In the synthesis of WO3, a predetermined amount of precursor, ammonium paratungstate was dissolved in deionized water and vigorously stirred at 80 °C, while a warm, concentrated HNO3 was added in drops. The solution mixture was kept at the temperature for about 1 h under continuous stirring after which the precipitate was left to settle for about 24 h at room temperature. A large amount of water was added followed by stirring for about 15 min to wash the precipitate and finally the mixture was left overnight to settle down before decantation of the liquid. The washing process was carried out twice after which the precipitate was removed by ultra-filtration using a polymer membrane with 0.48 μm pore size. The precipitates were dried at 100 °C overnight and then calcined at 600 °C for 2 h with ramp rate of 5 °C/min. For the preparation of In2O3 a predetermined amount of In(NO3)3·xH2O was dispersed in ethanol and stirred until dissolved. This solution was then added to a flask containing SBA-15 and stirred at room temperature for about 4 h, the weight ratio of In(NO3)3·xH2O to SBA-15 is 2:1. The thoroughly mixed solution was then filtered to recover the solid; the residue was then transferred to furnace in a crucible and dried at 45 °C overnight with temperature increment rate of 1.0 °C/min.
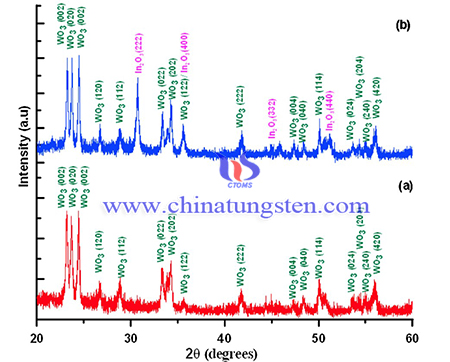
The Indium precursor/SBA-15 powder was then transferred into a quartz glass bottle where it is thermally decomposed at 600 °C for 4 h with temperature increase rate of 1.0 °C/min from the room temperature. The silica template SBA-15 was removed by dissolving the preformed In2O3/SBA-15 composite with 20 g NaOH in 240 mL and etched at 75 °C for 24 h. The indium oxide material was washed to neutral with deionized water, recovered by centrifugation and finally dried at 60 °C overnight, a light yellow product was obtained and transferred to a bottle. Finally In2O3-WO3 nano-composite was synthesized by direct mixing. The solid mixtures were well-grinded in mortal and pestle in equal ratio followed by heat-treatment in a sealed container at 550 °C with ramp rate of 2 °C/min for 5 h.
In conclusion, WO3/In2O3 nano was synthesized and used as a photocatalyst in the photo catalytic reduction of CO2 into methanol in the presence of the laser radiation of 355 nm wavelength. The maximum yield of methanol in this reaction is 496 μmol g−1 h−1 and the quantum efficiency and conversion efficiency are 2.25% and 12% respectively which is considered substantial compared to the data known.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com