Photocatalytic TiO2/WO3 by Spray Drying with Energy Storage Ability from Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Thursday, 31 December 2020 17:12
Titanium dioxide (TiO2) photocatalysis using sunlight as an irradiation source is a feasible technique to degrade pollutants in air. Although TiO2 possesses enticing properties such as chemical stability, strong oxidizing power, photo-resistance, availability, and low cost, its photocatalytic activity remains insufficient for large-scale applications.
Coupling TiO2 with WO3 is exceptionally appealing because of the physico-chemical properties of WO3: its band gap is relatively short (2.6–2.8 eV), thus requiring longer wavelengths for the excitation. Moreover, it is chemically stable, resilient to photocorrosion, and W exists in 2+, 3+, 4+, 5+, and 6+ oxidation states enabling it to store photogenerated electrons.
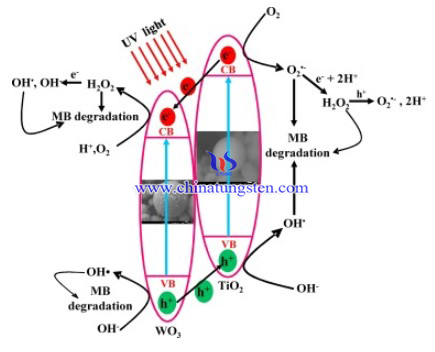
Thus, a synthesis method of efficient TiO2/WO3 composite powder was conducted, by applying a facile method via sol–gel (TiO2) and crash precipitation (WO3), followed by spray drying. Ammonium paratungstate (APT) was used as the starting material and source of tungsten. The hybrid catalysts are not only photocatalytically active, but also have photoinduced charge storage ability in the dark.The fabrication process of TiO2/WO3 photocatalyst are as below:
Titanium butoxide (Ti(oBu)4, ≥ 97.0%), formic acid (≥ 95%), ammonium paratungstate hydrate (APT, 99.99%) and Commercial Alcohols were received without further treatment.
TiO2 was synthesized by a simple sol–gel method. Titanium butoxide, anhydrous ethyl alcohol, formic acid and water were mixed for one hour at room temperature (molar ratios 60:500:27:1500). Anhydrous ethyl alcohol dissolved titanium butoxide under stirring, followed by the drop-wise addition of water and formic acid. Instantaneous hydrolysis occurs in the reaction mixture yielding a milky sol of hydrated titanium hydroxide.
We simultaneously synthesized WO3 by a crash precipitation technique. The molar ratio among APT, hydrochloric acid (HCl) and water was 0.005:200:500. APT was dissolved in HCl under stirring; afterwards the solution was added rapidly to water, resulting in the crash precipitation of a yellow white precipitate of WO3. We removed most of the water from the precipitate and then washed it several times with deionized water (4 × 100 mL). Finally, we added 20 mL of deionized water to the precipitate under constant stirring to obtain the WO3 suspension.

In a second step, we added the WO3 suspension to the stable sol of titanium hydroxide and stirred the hybrid suspension for 3 h before spray drying it. In the final step the dried powders were annealed at a temperature of 600 °C in a muffle furnace for 2 h to produce the crystalline hybrid TiO2/WO3 material.
We prepared a set of TiO2/WO3 powders by varying the WO3 precursor molar ratio at 0.025, 0.05, 0.075 and 0.1. The final material was denoted by TWx, where x is the molar ratio of WO3 precursor. The as-prepared products were characterized by XRD, N2 physisorption, FTIR, Raman, XPS, SEM-EDX imaging, photoluminescence, and time resolved photoluminescence (TRPL) via fluorescence.
In conclusion, TW0.075 was found to be best ratio to obtain the product with best photocatalytic properties and energy storage in the dark. In the photocatalytic degradation of methylene blue (MB) in liquid phase hybrid samples were the most active: TW0.075 degraded 90% MB in 100 min (30 min dark + 40 min light + 30 min dark), while W was least active with 70% MB degradation. TW0.075 was also the most active sample in the dark, degrading an extra 10% MB after the light went off. TiO2 and WO3 act in synergy. TiO2 coupled semiconductor photocatalysts with enhanced photocatalytic activity and greater electron storage ability.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com