Ammonium Paratungstate Applied in WO3–TiO2 Nanotube Photoelectrodes
- Details
- Category: Tungsten Information
- Published on Wednesday, 30 December 2020 01:31
As the fossil fuels brings about global warming and climate change, find a substitute source of clean energy such as wind energy, hydrogen energy, and solar energy has become critical. Photoelectrochemical (PEC) water-splitting technology is one of the most considerable technologies to synthesis H2 gas as a clean energy. Titanium dioxide (TiO2) has caught a lot attention as an effectual photoelectrode in PEC water-splitting for H2 production owing to its unique and promising functional properties, such as high photocatalytic activity, long term photo-stability, superior oxidation ability, inertness to chemical environment, as well as low cost.
Nevertheless, the drawbacks of such photoelectrode include the presence of defects or trapping sites, more grain boundaries, and disordered contact areas between two particles or spheres. In recent years, hybrid tungsten trioxide-titanium dioxide (WO3–TiO2) photocatalysts have gained considerable attention because of the interesting and unique features of the resultant binary oxide. WO3 can offer a relatively small band gap, strong absorption within solar spectrum, high optical modulation, long-term stability and high chemical stability over a wide pH range in aqueous solutions under evolving oxygen conditions.
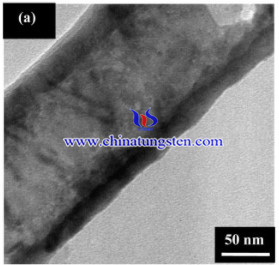
Thus, A preparation method of WO3–TiO2 nanotube photoelectrodes has been introduced through simple anodization and wet impregnation for the improvement of PEC water-splitting hydrogen evolution. The experimental procedures are as below:
- Titanium (Ti) foil (99.6%, 0.127 mm thick) was used in the present study. The Ti foil was cut into the desired dimension (50 mm × 10 mm) and then placed in ethylene glycol with 5 wt% ammonium fluoride (NH4F) and 5 wt% hydrogen peroxide (H2O2). During anodization, a small amount of H2O2 (≈0.5 mL) was added into the electrolyte at 10 min intervals. This composition was selected because it favors the formation of well-aligned and highly ordered TiO2 nanotube arrays. Anodization was performed at a constant potential of 60 V with a current density of 10 mA/cm2 using a Keithley DC power supply for 1 h, with Ti foil as the anode and a platinum rod as the cathode. The as-anodized Ti foils were washed with distilled water and then dried in a nitrogen (N2) stream.
- WO3–TiO2 nanotubes were prepared through wet impregnation using ammonium paratungstate (APT) as the precursor. Pure TiO2 nanotube foil was dipped into a 0.3 mM APT aqueous solution at different soaking times (i.e., 2, 3, 4, and 5 h). Based on our previous study, the molarity of the APT aqueous solution was fixed at 0.3 mM to produce WO3–TiO2 nanotubes with higher PEC water-splitting performance. The samples soaked in the APT aqueous solution were rinsed in deionized water and then dried in a stream of N2. Subsequently, the samples were annealed at 400 °C in an argon atmosphere for 4 h, which decomposed the APT into WO3.
The surface and cross-sectional morphologies of the samples were observed via field emission scanning electron microscopy (FESEM) using a Zeiss SUPRA 35VP at a working distance of approximately 1 mm. The elemental analysis of the W element incorporated into pure TiO2 was determined using energy dispersion X-ray (EDX) equipped in the FESEM. The morphologies were further confirmed through transmission electron microscopy (TEM) using an FEI CM 12 transmission microscope. The phase determination of the anodic layer was determined by X-ray diffraction (XRD) using a Philips PW 1729 operated at 45 kV and 40 mV. Photoluminescence (PL) and Raman spectra were obtained at room temperature using an LS 55 luminescence spectrometer (Jobin-Yvon HR 800UV). Photoelectron spectra were obtained through X-ray photoelectron spectroscopy.
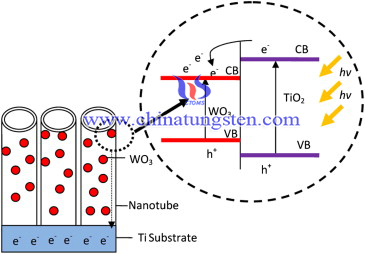
In conclusion, hybrid WO3–TiO2 nanotube photoelectrodes were successfully prepared using simple anodization and wet impregnation techniques. The soaking time in the W precursor APT aqueous solution plays an important role in the morphological control and content of WO3 species diffused into the TiO2 nanotubes as well as in the PEC water-splitting performances. A small content of WO3 species (after soaking for 2 h) significantly improved the PEC water-splitting performances by approximately 1.5 times higher than that of the pure TiO2 nanotubes. The formation of W–O–Ti bonding within the lattice of the WO3–TiO2 nanotubes acted as an efficient separator of the photo-induced charge carriers under visible illumination because of the heterojunction characteristics of WO3 and TiO2. By contrast, the excess amount of WO3 species in the TiO2 nanotubes formed an independent layer on the wall surface of the nanotubes, resulting in more recombination centers and poor PEC water-splitting performances.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com