Ammonium Paratungstate Applied in Nanostructured Molybdenum Carbide and Tungsten Carbide
- Details
- Category: Tungsten Information
- Published on Friday, 13 November 2020 01:48
Molybdenum carbide (Mo2C) and tungsten carbide (WC) have attracted attention for nearly four decades, due to their noble metal-like behavior in heterogeneous catalysis. These materials are catalytically active in reactions such as hydrocarbon hydrogenolysis and isomerization, ammonia synthesis, methanation, methane reforming and water–gas shift. Existing strategies for preparing high surface area carbides frequently produce non-stoichiometric surfaces, containing either residual oxygen or excess carbon which can block the active surface. Thus, an effective method that can remove the surface carbon is necessary.

Here a preparation method Mo2C and WC samples with high surface areas and low surface carbon deposition has been introduced, using ammonium paramolybdate (APM) and ammonium paratungstate (APT) as received. A temperature-programmed reaction has been applied in this method. The production procedures are as follows:
Unsupported carbides were prepared by heating the precursors in a reducing, carburizing gas flow. A quantity of 1.2 g APM) or APT (99.99%) was loaded into an alumina boat, which was then inserted into a vitreous silica tube inside a cylindrical furnace. MoO3 (from decomposition of APM at 350 °C in air) and WO3 (99.99%) were also tested as preparative precursors, for comparison with APM and APT. A gas mixture of 50%CH4/50%H2 was passed through the quartz tube at a rate of 50 cm3/min during the entire reaction. Molybdenum and tungsten precursors were carburized for 5 h at 650 and 900 °C, respectively, before being cooled to room temperature. The heating rate varied from 0.2 to 5 °C/min. Constant heating rates were adopted for most samples, while some samples experienced fast heating initially and slow heating when close to the final carburization temperature. Mo2C samples studied in this work were also prepared at 615, 700 and 900 °C. Some WC samples were prepared at 750, 800 and 950 °C as well.
After carburization, allof the samples were passivated in a 1%O2/95%N2 gas flow for more than 4 h before being removed from the silica tube, unless otherwise mentioned. Attempts were made to remove surface free carbon by heating the resulting carbides in 5%H2/95%N2 at 600 °C for 2 h, followed by passivation. Large samples (8–10 g) for constant-wavelength neutron study were made by combining different batches of samples prepared under the same conditions.
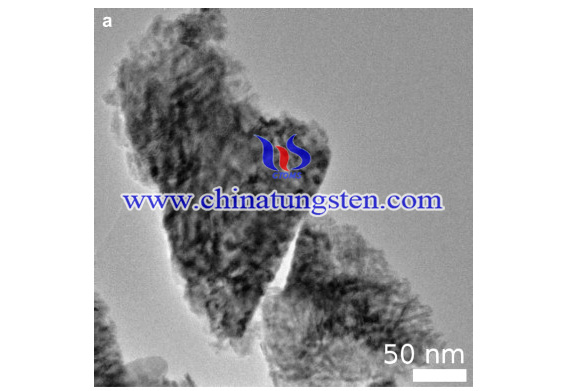
In conclusion, nanostructured Mo2C and WC with high surface areas and the desired surface compositions were prepared by temperature-programmed reactions using APM and APT as precursors. The crystal structures of the samples were explored by neutron diffraction, and the existence of graphite phases established by neutron pair-distribution function analysis. Electron microscopy reveals rather unusual surface morphologies in these carbides. The morphologies are very promising for catalysis, and we are now examining whether the morphologies are retained after being subjected to catalytic conditions.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com