Production Method of Nanostructured W–Co Powder by Thermal Reduction of Cobalt Doped Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Sunday, 14 June 2020 00:13
Tungsten carbide-cobalt (WC–Co) is an alloy of hard, ceramic tungsten carbide and the ductile cobalt, often known as cemented carbide. Some of the noteworthy properties of this alloy are strength, hardness, high electrical and thermal conductivity and high malleability and ductility.
The common preparation method of WC–Co material, is by reduction of tungsten oxides or ammonium paratungstate (APT) along with a carburization procedure to get tungsten carbide powder. The final product is on the micron scale. Recently, solution chemical synthesis methods such as spray conversion process and chemical co-precipitation have been introduced to the synthesis of nanoscale WC–Co material to improve the properties of WC–Co materials. WC–Co powders with Co concentration in the range 5–30% can be obtained in spray conversion processing.
A chemical co-precipitation synthesis method has been reported, where a suspension of APT and Co(OH)2 was reacted to synthesis W–Co starting material.
To illuminate the role of cobalt in the reduction process of the Co-doped APT precursor, and the influence of cobalt composition on the reduction of the W–Co precursor. A production method of nanostructured W–Co powder by thermal reduction of cobalt doped APT has been conducted:
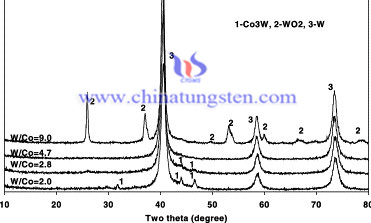
- Cobalt hydroxide and APT are prepared as raw materials. A chemical co-precipitation approach was used through a water mediated reaction between Co(OH)2 and APT to synthesize Co-doped APT precursors. The precursor powders were previously obtained after precipitation, filtration, and drying. The details of the precursor synthesis were described [6]. Four precursor samples with W/Co ratios of 2.0, 2.8, 4.7 and 9.0 were synthesized and noted as W20, W28, W47, and W90 respectively.
- The synthesized precursors containing water and ammonia were calcined at 220 °C for 5 h in nonflowing air. The calcined powders have mean particle size of around 250 nm, and contain a mixture of amorphous and crystalline phases. These powders still contain certain amount of water and ammonia. The heating rate of the samples of 5 °C/min from room temperature to the reduction temperature was used. We did not use a push type oven because a sudden heating of the sample leads to vigorous evaluating of ammonia and water. We also want to get homogeneous heating on the samples, remove water and ammonia and make the powders have a time to decompose during heating. To examine reduction during heating process, the sample was first heated up from room temperature to 600 °C, and then kept at this temperature for 1min under hydrogen atmosphere. The samples were also reduced in the furnace for a period of 1, 15, 30 min at 650 °C using the same heating rate to compare reduction rate of powders with different cobalt contents. The powders were filled in the tray with a size of 80 15 1 0 mm3, and the reduction process was carried out in a tube furnace. Pure hydrogen was used as reduction atmosphere with a flow rate of 400 cm3/min.
To sum up, completely reduced powders were well nanostructured with an average particle size between 20–50 nm. It has been discovered that the addition of cobalt results in a higher reduction rate toward the metallic phases. Moreover, the particle size of the reduced powders increases with increasing cobalt content, while its relative density decreases. The specific surface area of reduced powders at 650 °C increases for short reduction times, due to reduction of oxides to metal powder, and decreases for long reduction time due to agglomeration of metal powder. Cobalt plays the role as a catalyst during the reduction processing. On the other hand, it promotes the agglomeration of small particles.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com