Silver Tungstate Photocatalyst Preparation
- Details
- Category: Tungsten Information
- Published on Thursday, 06 June 2019 10:34
When a semiconductor irradiates light with energy above bandgap, the electrons of valence band excite in the conduction band, generate holes in the valence band, and generate electrons in the conduction band. They have strong oxidation and reduction abilities respectively, and have redox effects on molecular species in contact with semiconductors. Such an action is called photocatalysis, and such semiconductors are called photocatalysts.
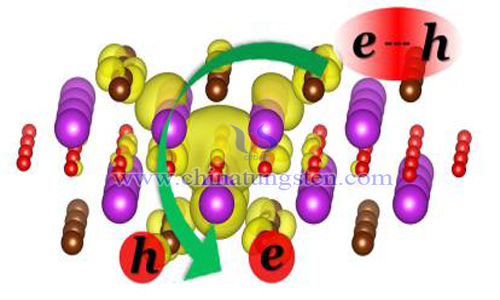
Tungsten oxide is a semiconductor photocatalyst with visible light response. Tungsten oxide particles can absorb most of the visible light occupying the room space. However, because the conduction band is located at the energy level of Oxygen-specific oxidation-reduction, oxygen can not be reduced in the electrons stimulated by the conduction band, and the amount of reactive oxygen species is insufficient. Therefore, tungsten oxide can not show high photocatalytic activity in the environment of visible light irradiation but not ultraviolet light irradiation. The preparation of silver tungstate photocatalyst by loading silver on tungsten oxide requires the following steps:
(1)Under stirring conditions, the dilute nitric acid solution was obtained by adding 5 ml concentrated nitric acid droplets with 65% mass fraction into 25 mL deionized water and stirring for 10 minutes; then the dilute nitric acid solution was mixed with 10 mL Na2WO4·2H2O solution to form a light yellow solution, stirred for 1 hour, and the slurry was obtained; finally, the slurry was transferred to a 50 mL stainless steel autoclave and hydrothermal reaction was 3 small at 180 ℃. After cooling, centrifugation, deionized water and ethanol cleaning, and drying, tungsten trioxide nanosheets were obtained, named WO3.
(2)Dispersion of 305.5 mg tungsten trioxide nanosheets in 15 mL deionized water by ultrasound for 0.5 h resulted in tungsten trioxide dispersion.
(3)50 mg silver nitrate was dissolved in 4 mL deionized water, then the obtained silver nitrate solution was added to the tungsten trioxide dispersion obtained by step (2) and agitated for 0.5 h under dark conditions to obtain Ag+WO3 dispersion.
(4)Silver tungstate photocatalyst was prepared by adding 5 mL sodium bicarbonate solution with 0.05 mol/L to Ag+WO3 dispersion solution obtained by step (3) drop by drop. After stirring for 2 hours, silver tungstate photocatalyst was obtained under xenon lamp with 100 mW cm2- light intensity for 10 minutes.
Silver tungstate photocatalyst has the advantages of high photogenerated electron-hole separation efficiency, high photocatalytic activity, strong redox ability, good stability and corrosion resistance. It has the advantages of simple synthesis method, low cost of raw materials, low energy consumption, and can be fused with other materials such as titanium dioxide.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com