Preparing Tungsten Trioxide Nanosheet with Electrochemical Anodic Oxidation
- Details
- Category: Tungsten Information
- Published on Friday, 10 August 2018 17:58
Tungsten trioxide and its hydrated oxides are important n-type semiconductor materials with a band gap between 2.4 and 2.8 eV. They have excellent physicochemical properties and unique electrocatalytic activity, and are applied in the fields of electrochromic, gas sensing, electrocatalysis, and photocatalysis.
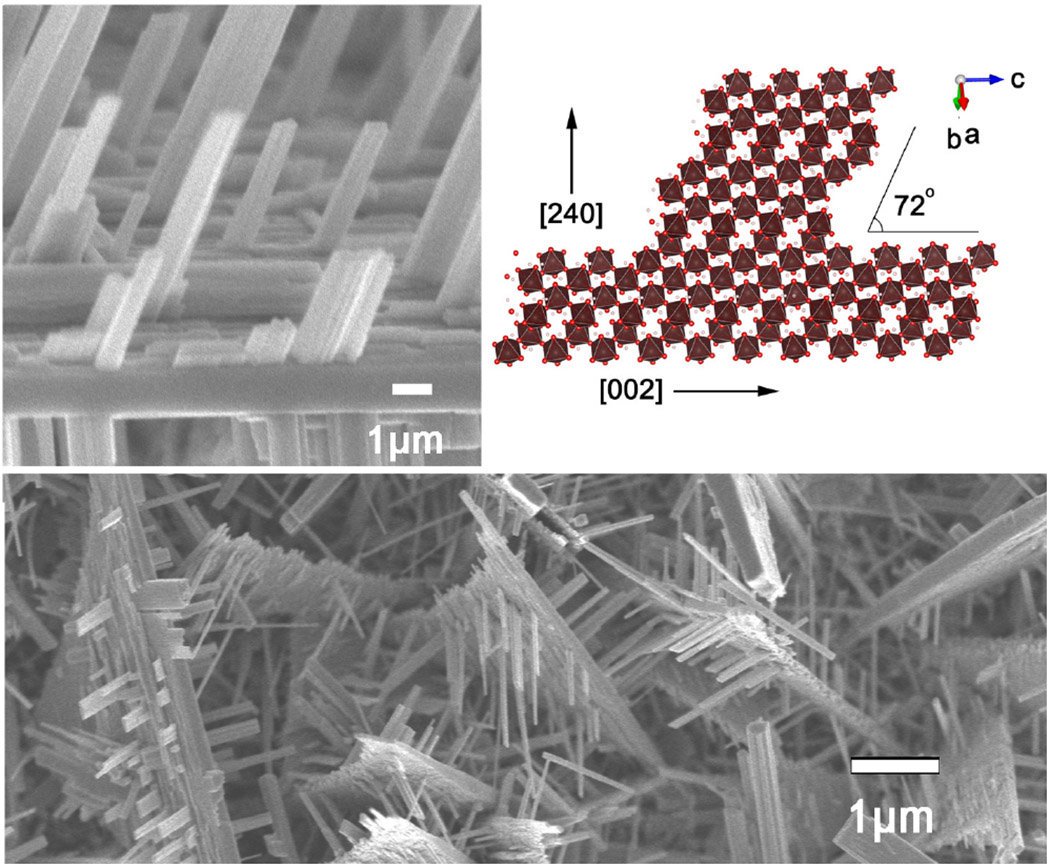
The tungsten trioxide nanomaterial with a sheet structure can effectively expose more catalytic active sites and reduce photogenerated holes due to its high specific surface area, rapid electron transport, and short diffusion path of photogenerated holes. Electron recombination extends the lifetime of photogenerated carriers and increases catalytic activity. In recent years, the preparation of tungsten trioxide nanosheets has become a research hotspot.
Electrochemical anodic oxidation is a low-cost method for constructing regular nanomaterials over a large area. The basic principle is to place a metal or alloy in the corresponding electrolyte as an anode, under specific conditions and the application of an electric field. Under the ion etching, the metal surface of the anode gradually accumulates on the surface of the electrode into a metal oxide film layer with a certain morphology and structure. However, the film layer usually formed is thin and has a single structure, and the utilization rate of the metal anode is very low, and the industrial production application does not have high economic benefits.
The process of preparing tungsten trioxide nanosheets by electrochemical anodic oxidation method is very simple: the tungsten sheet with thickness of 0.085mm is cut into 1×1cm2 specifications, ultrasonically cleaned in acetone, ethanol and deionized water for 15min respectively, rinsed with deionized water. Drying, to obtain a de-oiled metal tungsten electrode; adding ionic liquid 1-ethyl-methylimidazolium tetrafluoroborate ([EMim]BF4) 0.6 mL and deionized water 12 mL to ethylene glycol 47.4 mL The electrolyte was obtained (wherein the ionic liquid volume fraction was 1% and the deionized water volume fraction was 20%). Taking tungsten sheet as anode, titanium sheet (thickness 0.2mm, size 2×2cm2) as cathode, placed in electrolyte, keeping electrode spacing 1.5cm, controlling electrolyte temperature at 25°C, oxidizing to tungsten at 20V Dissolved and oxidized into powder (8h); collected solid powder, washed and deionized water, filtered, and dried to obtain a product of tungsten trioxide nanosheets.
Based on metal tungsten, a tungsten trioxide nanosheet powder is prepared by electrochemical anodic oxidation in an organic solvent electrolyte containing ionic liquid, by controlling electrolyte composition, reaction temperature, oxidation voltage and oxidation time to obtain tungsten oxide nanosheets of different size and structures. The prepared tungsten trioxide nanosheet has a regular structure, uniform size and large specific surface area, and the obtained tungsten trioxide nanosheet powder has high yield and stable properties, and has high utilization rate and high economic benefit for the metal tungsten matrix. It has broad application prospects in industrial applications.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com