Kinetics by Hydrogen Reduction of Tungsten Oxide
- Details
- Category: Tungsten Information
- Published on Monday, 05 February 2018 17:05
The kinetics of hydrogen reduction of tungsten oxide include the major thermodynamic changes during the reaction. Thermodynamic analysis of the oxidation of tungsten-hydrogen reduction shows that the reaction between tungsten oxide and hydrogen is rather complicated.
When the hydrogen reduction of tungsten oxide satisfies different thermodynamic reaction conditions, changing the different kinetic conditions will make the reaction process between tungsten oxide and hydrogen completely different. At the same time the size of tungsten powder produced will also be affected, and even the morphology of tungsten powder has greatly changed. The kinetic conditions of hydrogen reduction reaction most affecting tungsten oxide are reaction temperature, heating rate and carrier gas flow rate.

Due to the higher melting point of tungsten oxide and elemental tungsten, no phase change occurs during the reduction reaction. However, as the temperature increases, the volatility of tungsten oxide increases. And easily with the same reaction product of water molecules combined with tungsten oxide hydrate attached to the reactant surface, while affecting the reaction rate and reduction rate, or even to promote the growth of tungsten powder particle size. According to the calculation and theoretical analysis of the hydrogen reduction kinetics of tungsten oxide, it can be preliminarily considered that the hydrogen reduction reaction system of tungsten oxide is mainly controlled by the diffusion reaction step, which indicates that the reaction rate can be described as:
Where kint is the rate constant of the interface control reaction and t is the time. Hydrogen gas is required to react uniformly from the surface of the tungsten oxide particles to the interior of the particles in the public notice, and all the particles have the same size and morphology. The relationship between the radius of the particles in the geometric shrinkage model and the reaction order can be described as:
Where φ is the reaction fraction. r and r0 are the radii of the unreacted part and the whole particle, respectively, and d is the dimension. The particles here are three-dimensional spheres, note d = 3.

- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



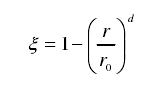

 sales@chinatungsten.com
sales@chinatungsten.com