Tungsten Carbide Forstner BitsⅠ
- Details
- Category: Tungsten Information
- Published on Tuesday, 24 November 2015 14:43
- Written by zhihua
- Hits: 365
Forstner bits are named for Benjamin Forstner, a 19th century American gunsmith who is credited with inventing them. Gunsmiths were the first to popularize the bit; they prized it for its ability to bore a smooth-sided hole in woodwork.
Tungsten carbide Forstner bits are a cylindrical drill bit used to bore precisely flat, deep holes into wood. It is particularly popular in furniture making and other large-scale woodworking projects in part because of its ability to drill deeply and precisely, even against the grain or through various inlays and joined pieces. They can cut on the edge of a block of wood, and can cut overlapping holes. Because of the flat bottom to the hole, they are useful for drilling through veneer already glued to add an inlay. They require great force to push them into the material, so are normally used in drill presses or lathes rather than in portable drills. Unlike most other types of drill bits, they are not practical to use as hand tools.
Forstner bits typically come in a range of different diameters, they are commonly available in sizes from 8–50 mm (0.3–2.0 in) diameter. Sawtooth bits are available up to 100 mm (4 in) diameter.

(To be continued. This article is divided into several parts. Here is part 1. For part 2 please refer to http://news.chinatungsten.com/en/tungsten-information/80701-ti-10455)
| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Wear of Coated Tungsten Carbide Tool In Cutting H.S. Steel Ⅻ
- Details
- Category: Tungsten Information
- Published on Tuesday, 24 November 2015 14:39
- Written by zhihua
- Hits: 330
In last part we get conclusions that the wear progress of tungsten carbide tool with (Al,Cr)N coating film is slower than the (Ti,Al)N coated one for the former one has higher hardness and higher oxidation temperature. And this part we go on to other conclusions based on the above experiments and analysis.
3) In addition, because the cutting temperature becomes lower due to the lower coefficient of friction of the (Al,Cr)N coating film, the wear progress of the (Al,Cr)N coated cemented tungsten carbide tool became slower.
4) In cutting hardened sintered steel with a (Al,Cr)N coated cemented tungsten carbide tool, there was little influence of the cutting speed on the tool wear within the range of the cutting speed from 0.50 m/s to 1.00 m/s.
As mentioned above, it was clear that the (Al,Cr)N coated cemented tungsten carbide tool is an effective tool material in cutting hardened sintered steel.

(The end-This article is divided into several parts. Here is part 12. For part 11 please refer to http://news.chinatungsten.com/en/tungsten-information/80698-ti-10452)
| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Wear of Coated Tungsten Carbide Tool In Cutting H.S. Steel Ⅹ
- Details
- Category: Tungsten Information
- Published on Tuesday, 24 November 2015 14:32
- Written by zhihua
- Hits: 327
In last part we know that The flank wear progress of tungsten carbide tool with (Al,Cr)N coating changes little with cutting speed from 0.5 m/s to 1.0 m/s. And this part we explain further the similarity of tool wear mechanism between the cutting speed of 0.50 m/s and 1.00m/s.
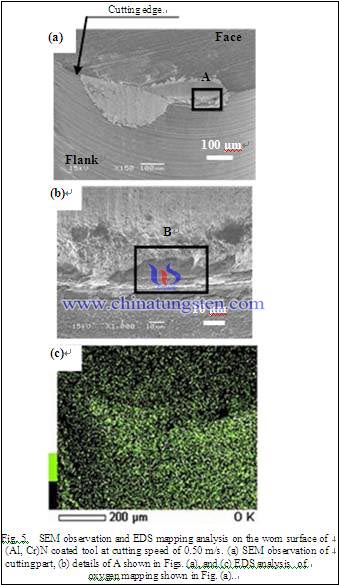
Fig. 5 shows the tool wear in the case of a cutting speed of 0.50 m/s. Fig. 5 (a) and (b) shows the SEM observation and Fig. 5 (c) shows the EDS analysis. Fig. 5 (b) shows the details of A shown in Fig. 5 (a). Fig. 5 (c) shows the EDS analysis in the case of the oxygen (O) mapping on the cutting part shownin Fig. 5 (a).
First, as compared with the wear of the tungsten carbide tool in the cutting speed 1.00 m/s shown in Fig. 1 (b) and that of the cutting speed 0.50 m/s shown in Fig. 5 (a), the tool wear of the cutting speed 0.50 m/s shows the same characteristics as that of the cutting speed 1.00 m/s. Next, as compared with the worn surface of the cutting speed 1.00 m/s indicated by “B” shown in Fig. 3 (ii)(a) and that of the cutting speed of 0.50 m/s indicated by “B” shown in Fig.5 (b), many striae scratched by any hard material are remarkably found on the worn surface in the case of both the cutting speed 1.00 m/s and the 0.50 m/s. Therefore, the main tool wear mechanism of the cutting speed 1.00 m/s and the 0.50 m/s is considered to be abrasive wear.
Finally, as compared with the oxygen element on the worn surface of the cutting speed 1.00 m/s shown in Fig. 3 (ii)(b) and that of the 0.50 m/s shown in Fig. 5 (c), the oxygen mapping on the cutting part shown in the cutting speed 0.50 m/s shows the same characteristics as that of the cutting speed 1.00 m/s. Therefore, the cutting speed is considered to have little influence on the cutting temperature within the range of the cutting speed from 0.50 m/s to 1.00 m/s.As mentioned above the cutting speed is considered to have little influence on the tool wear within the range of the cutting speed from 0.50 m/s to 1.00 m/s.
(To be continued. This article is divided into several parts. Here is part 10. For part 9 please refer to http://news.chinatungsten.com/en/tungsten-information/80632-ti-10439; for part 11, please refer to http://news.chinatungsten.com/en/tungsten-information/80698-ti-10452)
| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Wear of Coated Tungsten Carbide Tool In Cutting H.S. Steel Ⅺ
- Details
- Category: Tungsten Information
- Published on Tuesday, 24 November 2015 14:37
- Written by zhihua
- Hits: 334
In last part we know that the tool wear mechanism and cutting temperature of tungsten carbide tools in the cutting speed range from 0.50 m/s to 1.00 m/s are similar. And this part we come to get a final conclusion based on the above experiments and analysis.
4. Conclusion
In this study, to clarify the effectiveness of aluminum-chromium coating film for cutting hardened sintered steels, tool wear was experimentally investigated. The hardened sintered steel was turned with an aluminum-chromium based coated tungsten carbide tool according to a physical vapor deposition (PVD) method. Moreover, the tool wear of the aluminum-chromium based coated item was compared with that of the (Ti,Al)N coated tools.

(To be continued. This article is divided into several parts. Here is part 11. For part 10 please refer to http://news.chinatungsten.com/en/tungsten-information/80697-ti-10451; for part 12, please refer to http://news.chinatungsten.com/en/tungsten-information/80699-ti-10453)
| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Process for the Manufacture of Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Tuesday, 24 November 2015 09:07
- Written by xinyi
- Hits: 320
Ammonium tungstate, ammonium paratungstate, ammonium metatungstate or hydrated tungsten trioxide is produced by passing tungstate anions through an anion exchange membrane into an aqueous solution containing ammonium cations under the driving force of an electrical potential for a time sufficient to achieve a pH within the range in which the desired tungsten compound will form.
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |





 sales@chinatungsten.com
sales@chinatungsten.com