Humidity Sensor Using A Mixture of Ammonium Paratungstate Pentahydrate and Aluminium Sulphate
- Details
- Category: Tungsten Information
- Published on Thursday, 03 December 2015 09:57
- Written by xinyi
- Hits: 271
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Determination of Bismuth in High Purity Ammonium Paratungstate by Coprecipitation-Hydride Generation-Atomic Fluorescence Spectrometry
- Details
- Category: Tungsten Information
- Published on Thursday, 03 December 2015 09:54
- Written by xinyi
- Hits: 273
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Infiltration Time Effect on Tungsten Copper Electrode Properties III
- Details
- Category: Tungsten Information
- Published on Wednesday, 02 December 2015 16:20
- Written by xiaobin
- Hits: 264
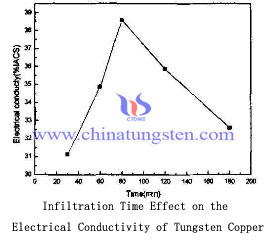
Viewed from the electrical conductivity of tungsten copper electrode, it is increasing with the infiltration time. We can learn from the graph that infiltration time from 30 min to 80min, the conductivity of tungsten copper alloy from 32.12% IACS to 38.6% IACS. While the infiltration time continues to increase, the conductivity was reduced from 38.6% IACS to 32.58% IACS.
This is due to the conductive properties of tungsten-copper depends mainly on the copper content and connectivity, as time increases, the amount of copper was infiltrated increases, more evenly distributed organizations tungsten copper, tungsten copper electrode conductivity correspondingly improved. But if infiltration time continued to increase, after that tungsten particles begin to grow up and there may arise organization copper pool, which causes the uneven distribution, so the hardness and conductivity of tungsten copper electrode will decrease.
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Annealing Process Effect on Tungsten Copper Electrode Properties
- Details
- Category: Tungsten Information
- Published on Wednesday, 02 December 2015 16:22
- Written by xiaobin
- Hits: 356
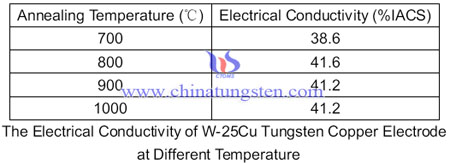
After infiltration cooling rate has a great impact on the copper layer quality, too fast cooling will be easy to produce pores and tiny cracks. Meanwhile, due to the furnace temperature unevenness, the copper layer thickness of products are not easy to be uniform, so it requires the use of a copper infiltrated after annealing treatment, which further improving the performance of a tungsten-copper electrode. Some experiments shows that annealing process has a great influence on the electrical conductivity of tungsten copper electrode, there is a table of the electrical conductivity of W-25Cu tungsten copper electrode at different temperature.
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Thermal Polycondensation of Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Wednesday, 02 December 2015 09:55
- Written by xinyi
- Hits: 254
Connected with the examination of the thermal polycondensation of ammonium paratungstate pentahydrate the chemical and morphological properties of intermediate phases formed during the thermal decomposition of APT have been investigated. We have studied the pH and the turbidity of the aqueous solutions of the intermediate phases, the solubility of the phases, and their rehydratation capability as well as the morphology of the crystallite granules and the grain size distribution. These properties of the original APT have been related to the same properties of the products of decomposition formed between different temperature ranges. The results obtained show unambiguously that each of the above mentioned properties suddenly changes in the temperature range 225 to 250°C. This temperature range coincides with the formation temperature of a new phase called APT II. The most probable formula of APT II is (NH4)8 [H2W13O43(OH)2]·H2O.
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |





 sales@chinatungsten.com
sales@chinatungsten.com