Formation of Heterotypic Substitutional Solid Solutions (NH4)10–xKx[H2W12O42] · n H2O in the Ammonium Paratungstate ‘Z'/Potassium Paratungstate ‘Z' System
- Details
- Category: Tungsten Information
- Published on Tuesday, 08 December 2015 10:31
- Written by xinyi
- Hits: 303
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Ammonium Paratungstate Tetrahydrate and Highly Pure Ammonium Paratungstate Tetrahydrate Method Producing
- Details
- Category: Tungsten Information
- Published on Tuesday, 08 December 2015 10:22
- Written by xinyi
- Hits: 274
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Co-doped Effects on Tungsten Copper Electrode II
- Details
- Category: Tungsten Information
- Published on Monday, 07 December 2015 16:30
- Written by xiaobin
- Hits: 303
Adding activate element to promote tungsten dissolution, and of the solid phase sintering and liquid phase diffusion densification during sintering tungsten particles dissolved in the liquid phase precipitation, round and accumulation, to obtain a high density. Its addition can remarkably improve the material properties, reduce the sintering temperature and shortening the sintering time.
However, the addition of these elements will be activated in a certain extent, reduce the thermal conductivity and electrical conductivity of tungsten copper electrode. Therefore, this kind of method is suitable for the occasions, which has lower demands of thermal conductivity and electrical conductivity. The effect of different content of Co on the density of tungsten copper electrode as follow:
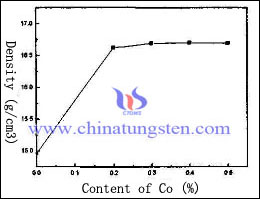
The graph shows that the reaction of tungsten copper electrode density is sensitive to the adding Co element, which increases at the beginning, 14.9g/cm3 rapidly increases to 16.6g/cm3. Afterwards, the content of Co increases constantly, the density of tungsten copper electrode changes a little and has a decreasing trend.
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Co-doped Effects on Tungsten Copper Electrode III
- Details
- Category: Tungsten Information
- Published on Monday, 07 December 2015 16:37
- Written by xiaobin
- Hits: 245
Sintering additives, which can dissolve tungsten, can be used for decreasing the porosity of tungsten copper electrode. The most common method is to add active elements, which takes advantage of active elements to dissolve tungsten and improve phase precipitation, round and accumulation, to obtain a high density. It can remarkably improve the properties of tungsten copper, decrease the sintering temperature and shorten the sintering time. The effect of different content of Co element on the hardness of tungsten copper electrode as follow:
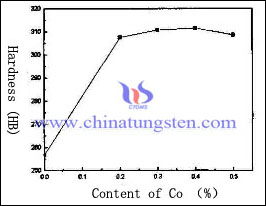
We can learn from the graph that the effect of Co element on the hardness of tungsten copper electrode is similar to the density of tungsten copper electrode, which remarkably increases at the beginning and tends to stabilization or decrease. When the content of cobalt is about 0.3%, the density and hardness of tungsten copper electrode reaches the maximum 16.69g/cm3, and HB311.6.
More infomations about Co-doped effects on tungsten copper electrode, click here:
http://news.chinatungsten.com/en/tungsten-information/81018-ti-10447
http://news.chinatungsten.com/en/tungsten-information/81083-ti-10458
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Thermal Decomposition of Ammonium Paratungstate Hydrate in Air and Inert Gas Atmospheres
- Details
- Category: Tungsten Information
- Published on Monday, 07 December 2015 09:50
- Written by xinyi
- Hits: 313
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
More Articles...
- Preparation of Coarse and Spherical Tungsten Powders by Ammonium Paratungstate
- Direct Solid State Synthesis of W–Al2O3 Nanostructured Composite Using Ammonium Paratungstate (APT) and Al Powder Mixture
- Growth and Morphology of W18O49 Crystals Produced by Microwave Decomposition of Ammonium Paratungstate
- Co-doped Effects on Tungsten Copper Electrode





 sales@chinatungsten.com
sales@chinatungsten.com