The Influencing Factors of Tungsten Copper Electrode Conductivity
- Details
- Category: Tungsten Information
- Published on Thursday, 10 December 2015 16:06
- Written by xiaobin
- Hits: 323
Tungsten copper electrode is one of the most widely used in Electrical Discharge Machining (EDM). Due to EDM uses pulsed spark between tool and workpiece, which ablate the metal by partial high temperature of discharging. Therefore, it is necessary that the electrode of EDM requires ablation resistance and excellent conductivity. What’s more, its distribution should be uniform.
Some researchers find that the measured conductivity is generally lower than the theoretical value by theoretical model of thermal conductivity value (also applied in analyzing conductivity). The influencing factors of conductivity of tungsten copper electrode are impurities, porosity and micro structure. Among them, impurity is the main factor, which trace will remarkably decrease the thermal conductivity and electrical conductivity of tungsten copper.
The source of impurities in the following areas: 1. activator, for improving the densification of tungsten copper, will form the solid solution with Cu and has an effect on the electrical conductivity. 2. The purity of raw material is lower, probably lead in impurities from outside during the preparation.
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Ni-doped Effects on Tungsten Copper Electrode III
- Details
- Category: Tungsten Information
- Published on Thursday, 10 December 2015 16:04
- Written by xiaobin
- Hits: 266
Except that the density and hardness of tungsten copper electrode, the effect of adding nickel (Ni) on the electrical conductivity of tungsten copper electrode is also can not be neglected. The effect of content of Ni on the electrical conductivity of tungsten copper electrode diagram as follow:
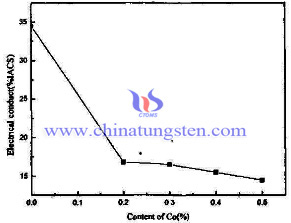
The diagram visually shows that the electrical conductivity of tungsten copper electrode dramatically decreases after adding nickel (Ni). (When the content of Ni is 0%, the electrical conductivity is 34.5%IACS, and the electrical conductivity decreases to 17.5%IACS after the content of Ni reaching 0.2 %.) Afterwards, with the increasing content of Ni, the electrical conductivity decreases constantly. The principle is similar to the adding cobalt (Co) that tungsten dissolved in nickel (Ni), which promoted the sintering densification. A large number of solid tungsten particles bonded contact with each other to form a continuous skeleton, limiting the role of liquid Cu infiltration, resulting in tissue tungsten copper alloy electrode uneven. In addition, external Ni atom enlarges the resistance through electrons of tungsten and copper scattering after adding active element Ni.
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Pure Ammonium Paratungstate from Tungsten Scraps
- Details
- Category: Tungsten Information
- Published on Thursday, 10 December 2015 11:05
- Written by xinyi
- Hits: 275

| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Ammonium Paratungstate Tetrahydrate X-Ray Powder Diffraction Data and Unit Cells
- Details
- Category: Tungsten Information
- Published on Thursday, 10 December 2015 11:34
- Written by xinyi
- Hits: 271
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Ni-doped Effects on Tungsten Copper Electrode II
- Details
- Category: Tungsten Information
- Published on Wednesday, 09 December 2015 16:03
- Written by xiaobin
- Hits: 244
Except cobalt (Co) element, Nickel (Ni) is also a kind of common additives, and the density, the hardness, the electrical conductivity and other comprehensive properties of tungsten copper electrode will be influenced by the content of Ni. According to the phase diagram of Cu-Ni and Ni-W as follow:
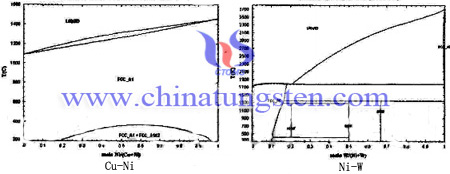
From the Cu-Ni and Ni-W phase diagram can visually see the nickel (Ni) and copper (Cu) is infinitely miscible, and tungsten W can be dissolved in Ni. Therefore, when tungsten copper composite powder mixed with Ni element, the porosity will decrease and the density and the hardness of tungsten copper electrode is improved. In addition, in the process of sintering, tungsten particles rearranged by the liquid Cu, which shortens the distance among the particles and further enhances the densification. Through SEM (Scanning Electron Microscope), we can find that the particle of skeleton is uniform and the granularity is finer. However, with the increasing content of Ni, the connectivity of tungsten skeleton will be better.
More infomations about Ni-doped effects on tungsten copper electrode, click here:
http://news.chinatungsten.com/en/tungsten-information/81132-ti-10471
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |





 sales@chinatungsten.com
sales@chinatungsten.com