APT Quote is Rising and Imports Declines
- Details
- Category: Tungsten Information
- Published on Wednesday, 06 January 2016 15:27
- Written by xinyi
- Hits: 263
The quote of APT is rising because the raw material tungsten concentrate is reluctant to sell, and it’s also rising faster than tungsten concentrate. In view of buying up not buying down, many APT manufacturers stopped quoting for the price to rise again. After the guide price was announced, APT price is rising rapidly, and there is basically no cheap shipping. It ‘s easy to see the determination of smelting factories, so the demand was not improved, but do not worry about the price to decline.
Powder market develops slowly in the initial stage, because the powder itself has a production receivables cycle, since APT manufacturers typically require cash spot, powder manufacturers borne more risks. Manufacturers worried that even if there is contract, after purchase of high-priced APT raw materials, if the market prices fell again when post, or if the buyer is not scheduled for payment or no delivery, etc., so the powder price increases slowly in the initial stage. After the guide price was quoted as 160 yuan / kg, driven by some manufacturers’ rapid price adjustment, the rest of the powder manufacturers also rise, making prices rise more than ten yuan, there is still a ascending trend.


| APT Supplier: Chinatungsten Online www.ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Sodium Metatungstate Application in Geology Field
- Details
- Category: Tungsten Information
- Published on Wednesday, 06 January 2016 08:54
- Written by linlu
- Hits: 300
Sodium metatungstate is also called hexasodium tungstate hydrate. It is a kind of white crystal. Molecular weight is 2968.01. The density is 3.1 g/ml and the refractive index is 1.5555. Under atmospheric pressure, sodium metatungstate is stable. But avoid contacting with the material and the oxide. Its sealing should be stored in ventilated and dry place at room temperature away from light. High density of sodium metatungstate, with non-toxic, easy preparation and recycled, are widely used in many fields, such as geology, soil science and Marine biology.

The application of sodium metatungstate in the field of geology mainly shows in separation of mineral and sedimentary rocks. It provides a new way of the fine grain of pure mineral separation. There are three main types of the micro granular single mineral separation: artificial selection, chemical separation and density of separation. The density separation method is simple and stable. But the commonly heavy liquid toxic has bad smell. Operation requirement for equipment is very high. The sodium metatungstate has many properties, such as non-toxic, easy preparation, high density and is widely used in geological and mineral separation industry laboratory assignments.
Multiple scientists have used sodium metatungstate to study the mineral separation. For example, Roz found sodium metatungstate added ferrosilicon can be used in the classification of mineral density, and this method is safe and simple. Using sodium metatungstate to separate and purify microgranular conodont has the advantages of safety, high efficiency and it’s easy to operate. With the application of sodium metatungstate in geological laboratory, mineral purification process will be safe, simple and stable. And sodium metatungstate also can be recycled to reach the purpose of saving cost and no secondary pollution. In addition, people also use sodium metatungstate to separate sedimentary rocks and analyze the organic matter in rock sediment. In this way, we can better study distribution, occurrence, mineral composition, chemical composition, structure, classification, origin, evolution history and metallogenic relationship of the rocks.
| Sodium Tungstate Supplier: Chinatungsten sodium-tungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Sodium Polytungstate Solution Preparation
- Details
- Category: Tungsten Information
- Published on Wednesday, 06 January 2016 08:45
- Written by linlu
- Hits: 1389
The molecular weight of sodium polytungstate is 2986.12 g/mol and relative density is 2.8. It’s soluble in water and is a new type of inorganic heavy liquid material produced by German SOMETU Company. It’s non-toxic, tasteless, neutral stable and easy prepared. It has the incomparable advantages of high recovery rate, use security with traditional heavy liquid. Relative density can reach 3.1 in particular. Many places used the original organic heavy liquid can be instead of sodium polytungstate. Within the scope of the pH 2 ~ 14, it’s stable and can be easily recycled. It is suitable for the separation of the vast majority of oxygen oxides and salts and has been widely applied in the separation experiment of fine mineral and conodont.
Because of good security and convenient recycling of sodium polytungstate, it’s very popular. Although its price in the international market, its prominent advantages make it be the first choice of the heavy liquid in numerous rock ore laboratory. However, the use of the heavy liquid should be paid attention to the following points:
1. Try not to contact with strong reducing agents, otherwise the solution will become blue and influence the separation effect of observation.
2. Should not be used to contain soluble or exchangeability calcium minerals, otherwise it’s easy to form calcium polytungstate precipitation, which limits its application in the clay mineral separation.
3. Under the condition of high relative density, heavy liquid viscosity increases. To reduce the separation time, use the centrifuge or choose larger size single crystal, as far as possible to speed up the separation process.
4. The operation is unfavorable to join too many samples at a time, in order to avoid congestion heavy export of funnel.
How to prepare sodium polytungstate? It can be divided into the following steps:
1. When preparing sodium polytungstate, distilled water or deionized water can be used, and glass, plastic or stainless steel container can be used.
2. Measure distilled water. According to the requirements of the density value, add sodium polytungstate to the water in the container in proportion. Keep stirring with a glass rod, so that it is fully dissolved.
3. The density of solution is measured with a hydrometer. According to the results, add distilled water or sodium polytungstate properly until suitable density value is reached.
4. Put the solution in the dust-free and closed environment to save.
5. You’d better measure the density of solution before use and make the appropriate adjustment in accordance with requirements.
| Sodium Tungstate Supplier: Chinatungsten sodium-tungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Sodium Polytungstate Separating-Purifying Zeolite
- Details
- Category: Tungsten Information
- Published on Wednesday, 06 January 2016 08:48
- Written by linlu
- Hits: 331
Zeolite is the generic terms of zeolite minerals and a kind of aluminum silicate minerals containing aqueous alkali metal or alkaline earth metal. It has unique structure, adsorption, ion exchange, catalysis and acid resistance properties. It belongs to a kind of fine minerals. Fine mineral particles are very small and surface area is large. There are great difficulties of the separation and purification. Especially zeolite separation and purification technology is not mature. It is difficult to achieve high purity. But through scientists' continuous efforts and many tests, finally using sodium polytungstate high-speed centrifugation achieves good effect.

The specific method of sodium polytungstate high-speed centrifugation is as follows:
1. Smash rock to less than 200 meshes by mortar and pestle. And then put finer samples in the glasses with secondary distilled water stirring well.
2. Use the particle-liquid suspension with ultrasonic 3 min ~ 5 min. Then put ultrasonic slurry into the centrifuge tube, with 2500 RPM speed centrifugal 3 minutes and 30 seconds. Pour out the supernatant fluid to remove part of less than 1 micron.
3. Then pour the supernatant fluid in another centrifuge tube. Repeat the above operation 5 times. In order to ensure that the vast majority of less than 1 micron particles has been removed, and before each centrifugal, it needs ultrasound to make larger particles suspended.
4. Pour out the supernatant fluid, air drying, greater than 1 microns particle into more than 2.3 g/cm3 sodium tungstate solution. Ultrasonic 3 min ~ 5 min to make particles dispersed into the centrifuge tube.
5. With 1100 RPM speed, the suspending liquid is centrifugal 33 min. After the centrifugal, zeolite minerals will float on the surface of the liquid and the heavier particles (quartz, feldspar and a few zeolite) will sink in the bottom of the tube.
6. Pour out a centrifugal about half of heavy liquid in the pipe, to remove the buoyancy of the zeolite, and make stick steel spoon in centrifuge tube wall of zeolite prepared in a centrifuge tube.
7. Fill two centrifugal tubes with secondary distilled water, suspended matter and heavy liquid and ultrasonic to fully mix, now heavy liquid density should be about 1.15 g/cm3.
8. With 1400 RPM speed centrifuge for 10 min. Particles should sink to the bottom. The heavy liquid into the glass can be recycled.
9. Use the second distilled water filling centrifuge tube to ultrasonic 2 min, with 3000 RPM speed centrifugal 5 min, zeolite particle coagulation. Wash 3 times repeatedly to completely wash sodium polytungstate. zeolite minerals becomes pale and not "sticky".
10. Put the zeolite in the fume hood to dry. Sometimes the surface of the zeolite "skin" is green or brown. Remove it. Then you can get more pure zeolite minerals.
| Sodium Tungstate Supplier: Chinatungsten sodium-tungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Behavior of Tin in Producing Ammonium Paratungstate 1/3
- Details
- Category: Tungsten Information
- Published on Tuesday, 05 January 2016 19:48
- Written by chunyan
- Hits: 366
In the production of ammonium paratungstate (APT) and tungsten smelting process, tin is extremely harmful in many impurities, and difficult to remove completely. Even a small amount tin can cause deadly in mechanical and physical properties, the necessity of removal is self-evident. According to GB/T10116-1988, mass fraction of tin in grade 0 APT should be less than 1 * 10-6, and in grade 1 it supposed no more than 3 * 10-6. With the reducing of tungsten concentrate, the mass fraction of impurities such as tin becoming higher and higher, and that makes APT production manufacturers great difficulty to keep quality stable. So it becomes very important to understand the behavior of tin in metallurgy.
Due to the complicated geological, different forms and proportion of tin exist in different tungsten ores. Different leaching rate is shown as below:
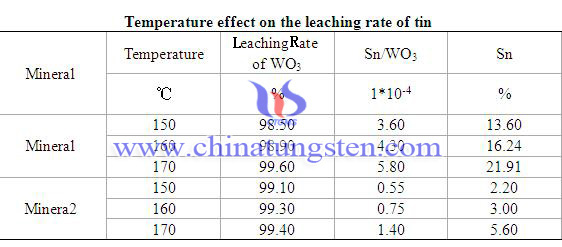

The most important minerals in tungsten metallurgical are tungsten scheelite and tungsten concentrate, cassiterite (SnO2) and tetrahedrite tin (Cu2FeSnS4) exist with them. Tungsten scheelite couldn't conduct electric, but SnO2 could. Tungsten concentrate has weak magnetism, however SnO2 hasn’t. SnO2 couldn't dissolve in acid and alkaline solutions, but, could react with molten sodium hydroxide and create sodium stannate which could soluble in water. Acid decomposition method is mainly used for tungsten scheelite. Usually use hydrochloric acid of density among 1.14~1.15, control temperature in 105-107℃. In the reaction process, tin exists in the state of cation, and gone with filtrate. What is worth to mention is that SnO2 don’t react with acids and leave in coarse tungstate. Then in the process coarse tungstate dissolves in ammonia, SnO2 isn’t reacting with ammonia, thus remained in Solid slag to separate with tungsten.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |





 sales@chinatungsten.com
sales@chinatungsten.com