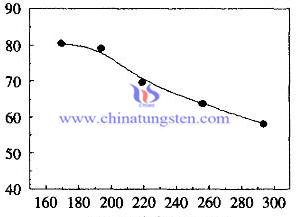
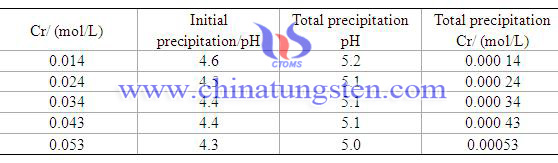
Chromium Behavior in Ammonium Paratungstate Crystallization Process 2/2
- Details
- Category: Tungsten Information
- Published on Wednesday, 06 January 2016 18:54
- Written by chunyan
- Hits: 235
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Chromium Behavior in Ammonium Paratungstate Crystallization Process 1/2
- Details
- Category: Tungsten Information
- Published on Wednesday, 06 January 2016 18:39
- Written by chunyan
- Hits: 251
(CH3COO)3Cr=(CH3COO)3 3-+Cr3-
Cr3-+3OH-=Cr(OH)3↓
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Nano Tungsten Trioxide Hydrothermal Method
- Details
- Category: Tungsten Information
- Published on Wednesday, 06 January 2016 17:36
- Written by qiongyao
- Hits: 253
 The methods of Hydrothermal and Water Bath belong to the solution method for preparing tungsten trioxide (WO3), it’s characterized by small experimental cost, low energy consumption, materials, degree of crystallinity and purity, which becomes the first choice for the preparation of WO3.The application of modern nano materials science and technology enhances the performance of the material, or shows some unexpected properties. The preparation of nano tungsten trioxide is based on the hydrothermal method for the experimental approach, nano and tungsten trioxide materials.
The methods of Hydrothermal and Water Bath belong to the solution method for preparing tungsten trioxide (WO3), it’s characterized by small experimental cost, low energy consumption, materials, degree of crystallinity and purity, which becomes the first choice for the preparation of WO3.The application of modern nano materials science and technology enhances the performance of the material, or shows some unexpected properties. The preparation of nano tungsten trioxide is based on the hydrothermal method for the experimental approach, nano and tungsten trioxide materials.
The preparation of cubic monoclinic nano particulate WO3, WO3 • H2Oand layered massive flower-like WO3 • H2O based on Na2WO4•2H2O as raw materials, CTAB and oxalic acid supplement aids, by hydrothermal method and the preparation of the water bath method. After the sintering process,WO3 • H2O changes WO3, and it keeps the original block and flower-like morphology. Three samples of the morphology of gas sensing tests show that the optimum operating temperature is 300 ℃, and flower-like structure shows the highest sensitivity, which shows the factors affecting the course of the reaction, mainly attributable to obtaining WO3 • H2O which is not easy to reunite and lay porous three-dimensional structure.
Research Indicates:
(1) Taking Na2WO4•2H2O as raw materials, CTAB and oxalic acid supplement aids in favor of changing the reaction rate, the reaction time decreases from more than 24h shortened to less than 8h.
(2) Alkaline hydrothermal method is also capable of producing nanometer tungsten trioxide product, the need to expand the pH ranges from 3.5 to 8.9.
(3) The obtained nano-tungsten trioxide has a difficult reunion and layered porous, the average particle size of the particles is about 1μm.
(4) Tungsten trioxide as additives products has better dispersion, and WO3 in gas sensing, electrochromic, chemical catalysis and electrochemical aspects show excellent performance.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Trioxide Doped Films Preparation
- Details
- Category: Tungsten Information
- Published on Wednesday, 06 January 2016 17:49
- Written by qiongyao
- Hits: 319
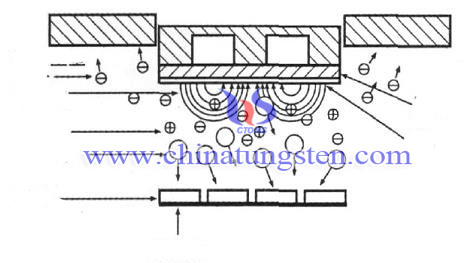 Tungsten trioxide (WO3) doped film is an excellent hydrogen sensitive material, it has been widely studied and applied because of its good electrochromic and gas electrochromic, photochromic, electrochemical properties, especially in the gas photochromic properties of gas sensors. In the catalytic precious metals platinum (Pt) palladium (Pd), etc., WO3film has good gasochromic effect to H2 at room temperature, which shows it is an important functional material.
Tungsten trioxide (WO3) doped film is an excellent hydrogen sensitive material, it has been widely studied and applied because of its good electrochromic and gas electrochromic, photochromic, electrochemical properties, especially in the gas photochromic properties of gas sensors. In the catalytic precious metals platinum (Pt) palladium (Pd), etc., WO3film has good gasochromic effect to H2 at room temperature, which shows it is an important functional material.
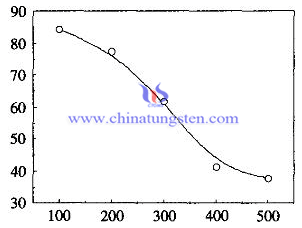
The temperature of tungsten trioxide film prepared by sol-gel method and the Combination of DC magnetron sputtering doped with Pd (or Pt) crystallization is higher than that of pure tungsten trioxide film. There are small amount of crystalline structure at 460 ℃ annealing treatment. The spectrophotometer test of results shows that: pure WO3 gel film has a high light transmittance (> 90%), but mixed with palladium or platinum thin film doped transmittance decreased significantly; and in the same condition of temperature, palladium-doped film doped is higher than the transmittance of the film . The transmittance of platinum-doped film sample increases by the annealing temperature at 100 ℃, 200 ℃, 300 ℃, 400 ℃, 500 ℃ .The film transmittance is gradually decreased, which is consistent with doped tungsten trioxide films.
The preparation of tungsten trioxide doped Pt (Pd) film based on the sol-gel method and the DC magnetron sputtering method, in the preparation of tungsten acid sol was added H2O2, ethanol can enhance the stability of the acid sol. When the molar ratio of H2O2 and tungsten acid is 1: 2, ethanol and acid volume ratio of 1: 2, the sol keeps long time, and the film plays a preservation effect. Tungsten trioxide surface becomes relatively flat after 100 ℃ annealing process, its molecules are tetrahedral structure. Tungsten trioxide surface becomes relatively flat after 400 ℃ annealing process, its molecules becomes cube structure too.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Harms of As,F in Ammonium Paratungstate Wastewater
- Details
- Category: Tungsten Information
- Published on Wednesday, 06 January 2016 17:28
- Written by xinyi
- Hits: 223

| APT Supplier: Chinatungsten Online www.ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |





 sales@chinatungsten.com
sales@chinatungsten.com