Ammonia Condensation in Ammonium Paratungstate Crystallization Affecting Factors
- Details
- Category: Tungsten Information
- Published on Thursday, 07 January 2016 20:20
- Written by chunyan
- Hits: 273
Condensation of ammonia tail gas from ammonium paratungstate is affected by many factors. Condensing often refers to the physical condensation process of the constant or high temperature gas, liquid cooled down, also reaction produced by polymerization of two or more physical. Studying the law NH3 saluted in water t different temperatures, and adopting appropriate technology and equipment will make a good effect on NH3 recovery. We analyze from the following 3 factors:
1. Area ratio of condensation and evaporation (Short for Sc/Se)
The concentration of ammonia decreased with the increasing of Sc/Se in APT crystallization. In the early evaporation stage, low temperature of the solution, high concentration of ammonia, much NH3 escaped and small amount of evaporation lead to higher concentration of ammonia; in the latter stages, temperature rising lead to ammonia concentration reduced, and less NH3 escaped, moisture evaporation, condensation of ammonia declining. When in the right Condensation strength, NH3 absorbed in water at maximum; the smaller Sc/Se is disadvantage to absorb ammonia gas; the larger Sc/Se will increase the condensation amount, resulting in the decrease of the concentration of ammonia.
2. Evaporation temperature
The saturation water vapor will increased with the evaporation temperature increasing, and the more water condensation will dilute ammonia, so low temperature evaporation is conducive to improve the concentration of ammonia.
3. Stirring speed
Evaporation of H2O and evaporation are the process of liquid→gas during the APT crystallization, and stirring can promote the concentration of ammonia.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Sodium Tungstate -Hydrogen Peroxide System Catalyzing-Degrading Methylene Blue
- Details
- Category: Tungsten Information
- Published on Thursday, 07 January 2016 19:55
- Written by linlu
- Hits: 258

| Sodium Tungstate Supplier: Chinatungsten sodium-tungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Sodium Tungstate and SLS Composite Corrosion Inhibition to Carbon Steel1/2
- Details
- Category: Tungsten Information
- Published on Thursday, 07 January 2016 19:46
- Written by linlu
- Hits: 252

| Sodium Tungstate Supplier: Chinatungsten sodium-tungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Sodium Tungstate and SLS Composite Corrosion Inhibition to Carbon Steel 2/2
- Details
- Category: Tungsten Information
- Published on Thursday, 07 January 2016 19:48
- Written by linlu
- Hits: 278
| Sodium Tungstate Supplier: Chinatungsten sodium-tungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Indirect Condensation Absorption Disposing Ammonia in Ammonium Paratungstate Crystallization
- Details
- Category: Tungsten Information
- Published on Thursday, 07 January 2016 18:20
- Written by chunyan
- Hits: 250
Ammonium paratungstate (APT) crystallization is the process of the polymerization of ammonia and acid. Ammonia (NH3) volatiles out from the solution the volatilized ammonia in the heating process. It will cause atmospheric pollution if ammonia discharged directly into the air. Ammonia combines with hemoglobin easily and destroys the function of oxygen transportation, even lead to death.
Ammonia, commonly known as NH3, is a colorless and tasteless gas, with a strong pungent odor, easily soluble in water, 1 volume of water can be dissolved 700 times the volume of ammonia under normal temperature and pressure. The environment department of China stipulates that waste water of tungsten smelting is the first class of elements to discharge the waste gas, zero discharge requirements is demanded. So, it is necessary for enterprises to take a new and efficient way of recycling tail gas in APT crystallization, in order to achieve the goal of zero emissions of ammonia gas.
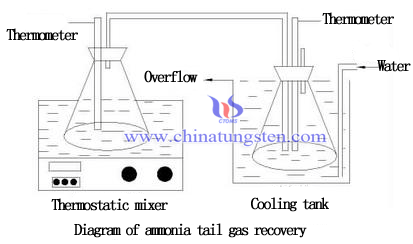
Indirect condensation method is an effective method for recovery of ammonia from APT tail gas. Two ways to recover ammonia produced from ammonium paratungstate crystallization.
1. Condensed into ammonia and returned to the reaction process;
2. Converted into NH4Cl and return to desorption of ion exchange process.
Compared the two ways, the front is of higher economic value. NH3 and water are polar molecules, ammonia and water molecules combining and becoming a hydrogen bond when NH3 soluble in water. With temperature increasing, the solubility of ammonia in water becoming lower, so the low-temperature condensation is an effective way to improve the recovery rate of ammonia.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |





 sales@chinatungsten.com
sales@chinatungsten.com