Shape Influence on CsxWO3 Nanocrystals Surface Plasmon Resonance
- Details
- Category: Tungsten Information
- Published on Tuesday, 05 April 2016 17:59
- Written by xinyi
- Hits: 343
Surface plasmons (SPs) are coherent delocalized electron oscillations that exist at the interface between any two materials where the real part of the dielectric function changes sign across the interface (e.g. a metal-dielectric interface, such as a metal sheet in air). SPs have lower energy than bulk (or volume) plasmons which quantise the longitudinal electron oscillations about positive ion cores within the bulk of an electron gas (or plasma). The charge motion in a surface plasmon always creates electromagnetic fields outside (as well as inside) the metal. The total excitation, including both the charge motion and associated electromagnetic field, is called either a surface plasmon polariton at a planar interface, or a localized surface plasmon for the closed surface of a small particle. The existence of surface plasmons was first predicted in 1957 by Rufus Ritchie. In the following two decades, surface plasmons were extensively studied by many scientists, the foremost of whom were T. Turbadar in the 1950s and 1960s, and Heinz Raether, E. Kretschmann, and A. Otto in the 1960s and 1970s. Information transfer in nanoscale structures, similar to photonics, by means of surface plasmons, is referred to as plasmonics.
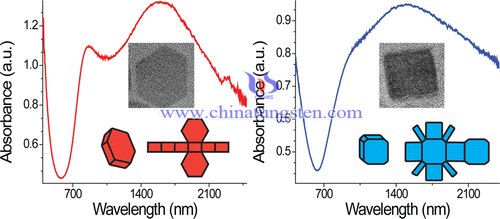
Localized surface plasmon resonance phenomena have recently been investigated in unconventional plasmonic materials such as metal oxide and chalcogenide semiconductors doped with high concentrations of free carriers. Synthesize colloidal nanocrystals of CsxWO3, a tungsten bronze in which electronic charge carriers are introduced by interstitial doping. By using varying ratios of oleylamine to oleic acid, synthesize three distinct shapes of these nanocrystals—hexagonal prisms, truncated cubes, and pseudospheres—which exhibit strongly shape-dependent absorption features in the near-infrared region. It's rationalized these differences by noting that lower symmetry shapes correlate with sharper plasmon resonance features and more distinct resonance peaks. The plasmon peak positions also shift systematically with size and with the dielectric constant of the surrounding media, reminiscent of typical properties of plasmonic metal nanoparticles.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
CsxWO3 Hydrothermal Synthesis and Near-Infrared Shielding Properties
- Details
- Category: Tungsten Information
- Published on Tuesday, 05 April 2016 17:50
- Written by xinyi
- Hits: 293

| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Cerium-Tungsten Trioxide SCR Denitration Catalyst 1/2
- Details
- Category: Tungsten Information
- Published on Friday, 01 April 2016 18:32
- Written by chunyan
- Hits: 289
 From the precious metal catalyst to today widely used SCR denitration catalyst which is using titanium dioxide as the main catalyst carrier, vanadium pentoxide, tungsten trioxide as main active ingredient, the SCR denitration catalyst has experienced a variety of important technological changes and condensed years of hard work from academics and numbers of plant personnel.
From the precious metal catalyst to today widely used SCR denitration catalyst which is using titanium dioxide as the main catalyst carrier, vanadium pentoxide, tungsten trioxide as main active ingredient, the SCR denitration catalyst has experienced a variety of important technological changes and condensed years of hard work from academics and numbers of plant personnel.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Cerium-Tungsten Trioxide SCR Denitration Catalyst 2/2
- Details
- Category: Tungsten Information
- Published on Friday, 01 April 2016 18:35
- Written by chunyan
- Hits: 291

| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Composite Carrier Tungsten Trioxide SCR Denitration Catalyst
- Details
- Category: Tungsten Information
- Published on Friday, 01 April 2016 18:30
- Written by chunyan
- Hits: 314
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |





 sales@chinatungsten.com
sales@chinatungsten.com