Running Time Affects Tungsten Trioxide SCR Denitration Catalyst
- Details
- Category: Tungsten Information
- Published on Wednesday, 20 April 2016 17:46
- Written by chunyan
- Hits: 260
 With the running time of tungsten trioxide SCR denitration catalyst going, the activity of the catalyst will be significantly reduced. The main reason is: catalyst poisoning, carbon and fly accumulation, sintering, active ingredient losing, as well as mechanical wear and destruction.
With the running time of tungsten trioxide SCR denitration catalyst going, the activity of the catalyst will be significantly reduced. The main reason is: catalyst poisoning, carbon and fly accumulation, sintering, active ingredient losing, as well as mechanical wear and destruction.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Defect of Tungsten Trioxide SCR Denitration Catalyst
- Details
- Category: Tungsten Information
- Published on Wednesday, 20 April 2016 17:44
- Written by chunyan
- Hits: 275
 SCR denitration technology has been used in coal-fired power plant for 30 years; it stands out as the efficient flue gas denitration technology, which is a reliable method of denitration. SCR technology can be divided into the three types of high (greater than 400°C), medium (among 300~400°C) and low (less than 300°C) temperature catalyst depending on the reaction temperature. Although tungsten trioxide SCR denitration catalyst is the most mature technology which has been constantly research, it still has a lot of defects.
SCR denitration technology has been used in coal-fired power plant for 30 years; it stands out as the efficient flue gas denitration technology, which is a reliable method of denitration. SCR technology can be divided into the three types of high (greater than 400°C), medium (among 300~400°C) and low (less than 300°C) temperature catalyst depending on the reaction temperature. Although tungsten trioxide SCR denitration catalyst is the most mature technology which has been constantly research, it still has a lot of defects.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
CCFL Functionally Graded Cemented Carbide (2/2)
- Details
- Category: Tungsten Information
- Published on Wednesday, 20 April 2016 15:42
- Written by xiaobin
- Hits: 237
Nitride process can form hardened layer with surface-rich cubic phase; while denitrification can form flexible layer of CCFL (cubic carbide free layer). Its mechanism is that the environment of nitrogen partial pressure of nitrogen balance sintered body partial pressure of nitrogen generated escape, which occurred in the formation of nitrogen and CCFL. Compared to the traditional structure of cemented carbide preparation process, the biggest difference between CCFL, the most critical part of functionally graded cemented carbide is that the sintering process.
Since the atomic diffusion rate in the liquid phase faster, CCFL typically select a liquid phase is formed at the stage of sintering becomes gradient and usually uses mixed atmosphere of argon and nitrogen to prevent nitrogen atom escape. Afterwards, we use micro-hardness method, nano-indentation method and XRD (X-ray diffraction) to measure the hardness, fracture toughness and stress of CCFL cemented carbide. The results show that the change in hardness is proportional to the content of binder phase, CCFL decrease in hardness, fracture toughness increased, and the overall stress in a weak state, which inhibits the cracks formation and expansion.
There are many factors of CCFL cemented carbide, such as sintering, carbon and nitrogen content and related compounds. With the extension of sintering time and the sintering temperature, CCFL thickness also increases and the rate of formation has been quickened; with the increase of nitrogen content, nitrogen has a larger range of activity, which CCFL driving force increased, the rate of formation improved; high carbon content can significantly increase the amplitude of the Co-rich, conducive to the formation of CCFL. In addition, the nitride and carbide content increases from zero, CCFL forming ability enhanced, increasing the thickness, but the solubility of nitrogen in the binder phase is limited, and when it is saturated at this time forming ability of CCFL is the best. After the increase nitrides, undissolved nitride takes longer to dissolve and diffusion will hinder the formation of CCFL.
| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Ion Diffusion Mechanism in Potassium Sodium Tungsten Bronze
- Details
- Category: Tungsten Information
- Published on Wednesday, 20 April 2016 17:34
- Written by xinyi
- Hits: 296
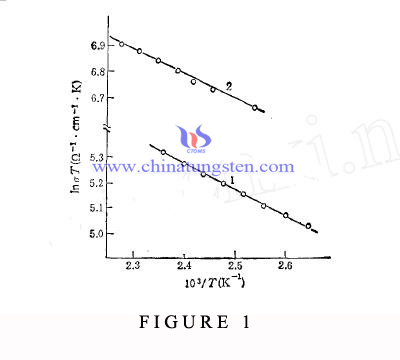
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
CCFL Functionally Graded Cemented Carbide (1/2)
- Details
- Category: Tungsten Information
- Published on Wednesday, 20 April 2016 15:40
- Written by xiaobin
- Hits: 239
Tungsten carbide, also called cemented carbide, is composed of hard phase WC and binder phase Co, which has higher hardness, strength and excellent wear resistance and has been widely used in metal cutting industries. In order to further improve the hardness and wear resistance of tungsten carbide cutting tools, in the actual production is often by adjusting the alloy binder phase content and add cubic carbides (such as TiC, TaC, Cr3C2, etc.) or the like by introducing coating technology (such as CVD chemical vapor deposition, PVD physical vapor deposition) higher in the cemented carbide substrate surface coated surface hardness, chemically more stable phase ceramic layer. However, there are some differences in the coefficient of thermal expansion and binding force between tungsten carbide matrix and coating layer, so the coating will crack and even peel off at work, which remarkably affects the using properties of tungsten carbide coated cutting tools and decreases the overall efficiency.
New functionally graded cemented carbide can effectively inhibit the formation and spreading of cracks to the substrate coating cracks and improve the reliability and service life of tungsten carbide cutting tools. From the component point of view, it can be divided functionally graded cemented carbide non-equilibrium carbon and nitrogen carbide. Unbalanced carbide or carbon, said carbon-poor carbide carburizing process can be prepared for two-phase drilling with carbide; The nitrogen-containing alloy can be divided according to the internal balance of nitrogen partial pressure difference between the pressure of the nitrogen balance in the environment can be achieved nitride, and nitrogen, and forming two distinct surface.

| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |





 sales@chinatungsten.com
sales@chinatungsten.com