Isostatic Pressing for Round Tungsten Bar
- Details
- Category: Tungsten Information
- Published on Tuesday, 24 May 2016 11:05
- Written by xuejiao
- Hits: 250
 Tungsten round bars formed by isostatic pressing can improve the uniformity of the tungsten material structure that can avoid the problem of brittle fracture on tungsten wire to some extent, thereby improving its wire-wounded performance, and improve the uniformity of wire diameter and improve the finished product rate, which has good reproducibility. When use square bar molds for making tungsten bars, the binder should be added to the doped tungsten powder, so that the internal forming tungsten is easily to cause stratification and likely to cause uneven density, cracks and other phenomena. Especially during the processing of tungsten square bars, the amount of deformation the four corners of tungsten bars is large, on the other hand, its center deformation is small, so its deformation is very unevenly at a high temperature, it is easy to produce localized cracking and final affecting the quality of tungsten wire and the finished product rate.
Tungsten round bars formed by isostatic pressing can improve the uniformity of the tungsten material structure that can avoid the problem of brittle fracture on tungsten wire to some extent, thereby improving its wire-wounded performance, and improve the uniformity of wire diameter and improve the finished product rate, which has good reproducibility. When use square bar molds for making tungsten bars, the binder should be added to the doped tungsten powder, so that the internal forming tungsten is easily to cause stratification and likely to cause uneven density, cracks and other phenomena. Especially during the processing of tungsten square bars, the amount of deformation the four corners of tungsten bars is large, on the other hand, its center deformation is small, so its deformation is very unevenly at a high temperature, it is easy to produce localized cracking and final affecting the quality of tungsten wire and the finished product rate.
In order to solve the problems of tungsten square bars, developing isostatic pressing way to form round tungsten bars. Hot isopressing (HIP) is a manufacturing process, theorized in the 1970s, used to reduce the porosity of metals and increase the density of many ceramic materials. This improves the material's mechanical properties and workability. During isostatic pressing, it is preferable to make certain provisions of the metal powder. Different shapes, particle size and distribution of particle size of metal powders have a significant impact on manufacturing process of tungsten bars. Therefore, average particle size of doped tungsten powder should be 2.5 ~ 5.0μm, the oxygen content is lower than 2500 × 10-6. At the time of preparation of tungsten powder, it is important to control the humidity. When the tungsten powder to be filled into a soft film sets, be sure to place the powder uniformly and intimately, do not leave any gaps, in order to ensure the quality of the final product. After the preparation is completed, the speed of the release should be slowly. But there are some problems in the process. In the high pressure liquid when pressed, the size and shape of the final product cannot be controlled. Therefore, it is better to use a metal powder automatic loading machine, so we can guarantee that the final tungsten bars are flat, smooth and uniform.
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
IF Furnace for Highly Purity Tungsten Bar
- Details
- Category: Tungsten Information
- Published on Tuesday, 24 May 2016 11:02
- Written by xuejiao
- Hits: 223
Most high-purity tungsten bars are formed by conventional vertical sintered manufacturing processes, this kind of traditional process can consume a lot of power. In addition, at the time of preparation, tungsten bar would be affected by hydrogen flow and voltage fluctuations. If the operators have insufficient professionalism or their operations have some differences in the mass-produced, which will result in different quality of products in the same batch. On the contrary, if sintering in the intermediate frequency (IF) furnace, it would not only cost the lower energy, but also has with high collection rate.
Chemical compositions of tungsten bars made by the two processes are the same. So, IF furnace sintering method can also produce highly purity tungsten bars. Compared to the traditional production method, the density and hardness of wolfram bars formed by medium frequency furnace are much higher, and distribution of its grain is uniform. Density distribution of each part of wolfram bar made by conventional sintering is parabolic state from the top down, the density of a tungsten bar has a difference of 0.37g / cm3. The density of a wolfram bar produced by IF firing only has a difference of 0.2 g / cm3, whose density distribution is more uniformity than the traditional one. Vertical furnace can only hold a tungsten bar, which would take away a lot of heat from surface because of the excessive hydrogen, resulting in the surface temperature of tungsten is lower than the core temperature. However, wolfram bar itself is heating element in medium frequency furnace, so that the core temperature will be higher than the surface temperature. Therefore, the grains of a sintered tungsten bar will be unevenly distributed. For IF sintering, it can be placed more tungsten bars which can be displayed closely, then the heat taken away by hydrogen will be relatively less. Therefore, the final products would have more uniform density distribution.
All in all, IF sintering not only has a capacity of producing a higher purity tungsten bar, but also ensures a stable quality for the manufacturing of goods in large quantities. Further, since the power consumption is low, the required cost is also less. IF sintering adopts eddy current sensor which can make crucible furnace heat and then radiation to the tungsten, so the outside and inside of tungsten bars are nearly the same, therefore the tungsten grains of cross-section are uniform.

| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Zr Affects Tungsten Trioxide Denitration Catalyst
- Details
- Category: Tungsten Information
- Published on Monday, 23 May 2016 17:09
- Written by chunyan
- Hits: 269
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
WO3/TiO2-ZrO2 SCR Denitration Catalyst
- Details
- Category: Tungsten Information
- Published on Monday, 23 May 2016 17:13
- Written by chunyan
- Hits: 255
 Denitration catalyst is the core part of the SCR process, the denitration catalyst applied in industrial mostly is vanadium/titanium based, with the temperature window of 370~430℃, and the denitration efficiency of about 80%. However, the vanadium/titanium (anatase) system is an unstable system, titanium dioxide is the metastable crystal with a smaller surface area; besides, when heated, the surface is easily shrank, and converted into stable thermodynamic state of rutile at a certain temperature and pressure, resulting in the declining of irreversible denitration efficiency. Experiments showed that the adding zirconium oxide can improve the tungsten trioxide denitration catalyst surface acidity, thermal stability and support denitrification efficiency.
Denitration catalyst is the core part of the SCR process, the denitration catalyst applied in industrial mostly is vanadium/titanium based, with the temperature window of 370~430℃, and the denitration efficiency of about 80%. However, the vanadium/titanium (anatase) system is an unstable system, titanium dioxide is the metastable crystal with a smaller surface area; besides, when heated, the surface is easily shrank, and converted into stable thermodynamic state of rutile at a certain temperature and pressure, resulting in the declining of irreversible denitration efficiency. Experiments showed that the adding zirconium oxide can improve the tungsten trioxide denitration catalyst surface acidity, thermal stability and support denitrification efficiency.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
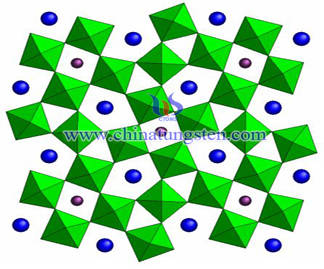
Ammonium Tungsten Bronze Nanoparticles Preparation
- Details
- Category: Tungsten Information
- Published on Monday, 23 May 2016 16:04
- Written by xinyi
- Hits: 267
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |





 sales@chinatungsten.com
sales@chinatungsten.com