Composite WO3/CdS/W Photocatalyst
- Details
- Category: Tungsten Information
- Published on Tuesday, 14 June 2016 17:42
- Written by chunyan
- Hits: 262

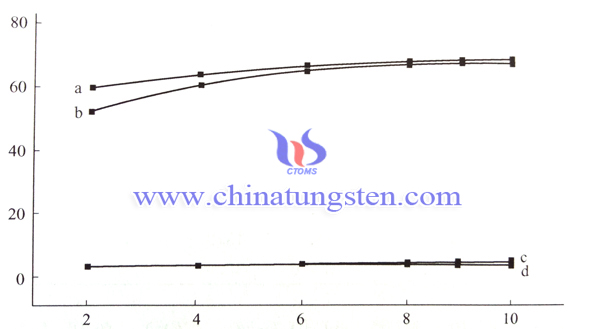
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
As-Reduced Ammonium Tungsten Bronze Nanoparticles Preparation
- Details
- Category: Tungsten Information
- Published on Tuesday, 14 June 2016 17:36
- Written by xinyi
- Hits: 290


| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Trioxide Denitration Catalyst Applies High Temperature Flue Gas Denitration
- Details
- Category: Tungsten Information
- Published on Monday, 13 June 2016 18:31
- Written by chunyan
- Hits: 242

| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Carbide Roll Ring
- Details
- Category: Tungsten Information
- Published on Tuesday, 14 June 2016 16:57
- Written by xiaobin
- Hits: 277
Compared with the roll ring by other materials, tungsten carbide roll ring has many advantages, such as high hardness, high flexural or compressive strength, with low affinity of steel, low coefficient of expansion and excellent wear resistance and so on. What’s more, it can also effectively accelerate the rolling process, remarkably decrease stopping times and maintain high-speed rolling, which improves the overall efficiency; roll ring does not occur substantially scratches, it burns the melt and stick onto steel, and the shorter the time required for grinding; the rolled products have high dimensional accuracy, good surface quality, overall performance has improved significantly. According to differences of materials, tungsten carbide roll ring can be specifically divided into WC-Co based carbide, TiC based carbide and steel bonded carbide. In addition, in order to meet the special requirement of wear resistance and corrosion resistance, it can correspondly add some Ni, Cr elements. Generally, the content of WC of tungsten carbide is between 70%-97%, decrease the binder Co or the grain size of WC will all improve the hardness of roll ring.
The monolithic cemented carbide roll ring, for the user, a one-time investment costs are relatively high, which is also a barrier of development of carbide roll ring. So consider on the properties and the cost, researchers develop new tungsten carbide composite roll ring, which can greatly save the carbide consumption and decrease the production cost. It is composed of tungsten carbide outer ring and ductile iron inner ring. Tungsten carbide outer ring can ensure the roll ring has no deformation by high-speed friction under high temperature and high pressure, so the quality and dimensional accuracy of products will not be affected; ductile iron inner ring has high strength, good strength and ductility, which play an important role in rolling force delivering and can effectively reduce the failure rate of roll ring under impacting. Furthermore, it can also be processed by ductile iron and keyway with the keyway by a plurality of taper roller ring (up to 4) mounted on the roller body, compared to the overall carbide roller ring with the roll change a lot of convenience.

| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
TTB Structures Lead-Free Ferroelectrics
- Details
- Category: Tungsten Information
- Published on Monday, 13 June 2016 18:20
- Written by xinyi
- Hits: 267

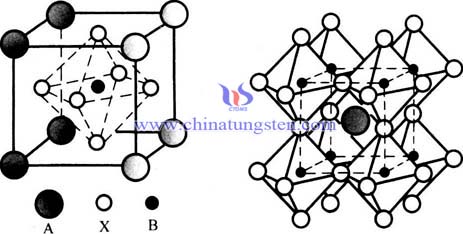
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |





 sales@chinatungsten.com
sales@chinatungsten.com