Tungsten Powder and Electrolytic Capacitor
- Details
- Category: Tungsten Information
- Published on Friday, 24 June 2016 18:11
- Written by xinyi
- Hits: 333


| Tungsten Powder Supplier: Chinatungsten Online tungsten-powder.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Carbide Button — Profile Optimization
- Details
- Category: Tungsten Information
- Published on Friday, 24 June 2016 18:09
- Written by xiaobin
- Hits: 268
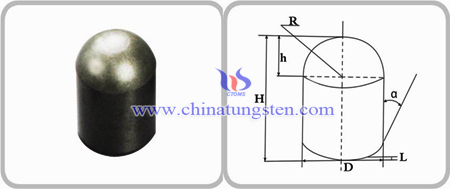
Most of tungsten carbide button profiles are round and bullet-shaped. But in the process of using, conventional round tungsten carbide buttons are prone to be passivated and affects the efficiency of drilling; bullet-shaped tungsten carbide button has tapered crown and small ball radius, so it will be broken easily because of the deficiency in matrix strength in the large impacting power and the hard rock formation. Therefore, tungsten carbide button profile has a significant effect on the performance, which button profile optimization becomes a direction of research.
Based on spherical stamper effective rock breaking mechanism and constant bending stress theory, domestic researchers design blunt-resistant button. It combines the advantages of round buttons and bullet-shaped buttons, the crown consists of spherical shape cap and approximate cone; Wherein the body is subjected to the spherical cap spherical stamper rock breaking, approximate cone dovetail die broken rock, broken rock dominated the first spherical stamper, the stamper wedge after breaking rock, giving the formation of a common rock-breaking effect. In addition, by analyzing the stress distribution of the rock under the spherical die and wedge die proved more rock-breaking effects resistant carbide ball blunt teeth, drilling speed faster, and better impact toughness, not easy to be passivated.
There are experimental data shows that adopt blunt-resistant carbide button, the life and average drilling rate is 36% and 28% higher than bullet-shaped buttons. Another optimized button is composed of fore super-hard sphere and secondary super-hard sphere, the fore super-hard sphere produces shear after breaking rocks; when the drill impacts the rock again, the secondary super-hard sphere impacts on the shear and breaks the rocks repeatedly. This makes carbide button drill bit when drilling footage longer need to drill and drill bit protrusion treatment, but also to maintain a good rock fragmentation capability and self-sharpening performance.
| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Oxychlorides
- Details
- Category: Tungsten Information
- Published on Friday, 24 June 2016 16:46
- Written by Cristina
- Hits: 422
Two oxychlorides of tungsten, respectively WO2Cl2 and WOCl4, are known.
Grades: 98%, 99.9%
Formula: WOCl4
CAS No: 13520-78-0
Form: orange / red crystalline powder
Tungsten dioxydichloride (tungstyl chloride), WO2Cl2, was originally considered to be a chloride of tungsten, and was obtained, although mixed with a certain amount of the oxytetrachloride, by interaction of chlorine with tungsten dioxide. In attempting to prepare tungsten hexa-chloride by the action of sulphur chloride upon tungsten trioxide, Bourion found that the product of the reaction was a mixture of the two oxychlorides only. Further, it is produced by heating tungsten hexachloride with oxalic acid.
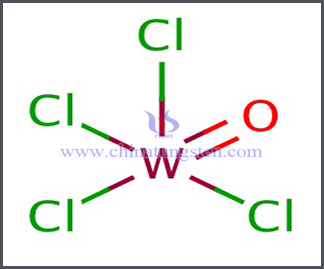
This oxychloride forms stable yellow crystals which readily sublime, but which also melt at 259° C; at much higher temperatures the substance largely dissociates into tungstic anhydride and the oxytetrachloride. Partial decomposition is also effected by warming with water. Tungsten oxytetrachloride, WOCl4, is obtained by heating tungsten pentachloride or hexachloride in oxygen, by heating the penta-chloride with oxalic acid, by interaction of phosphorus pentachloride with tungsten trioxide; together with the oxychloride, WO2Cl2, by heating tungsten trioxide in a current of sulphur chloride vapour, by heating the trioxide or oxychloride, WO2Cl2, in the vapour of the hexachloride alone or mixed with chlorine, or by passing dry chlorine over a heated mixture of tungsten trioxide and carbon, the main product being then the oxytetrachloride, which may be obtained in a moderately pure state by taking advantage of its greater volatility than that of the dioxydichloride. It is also formed, together with small quantities of the hexachloride and probably of the pentachloride, when carbonyl chloride is passed over heated tungsten powder.
Tungsten oxytetrachloride forms splendid transparent, ruby-red, needle-shaped crystals which melt at 210° C. and boil at 227.5° C, giving a red vapour of normal density. By water it is decomposed, giving first the yellow oxychloride, and further, hydrochloric and tungstic acids. A certain amount of the yellow oxychloride is also formed from it by loss of chlorine on sublimation in air or oxygen.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Carbide Button — Ultra-fine Crystallization and Gradient Optimization
- Details
- Category: Tungsten Information
- Published on Friday, 24 June 2016 18:07
- Written by xiaobin
- Hits: 256
Tungsten carbide button is a kind of rock drilling tools composed of hard phase WC and binder phase Co and has high hardness, high strength, high wear resistance and good impact toughness. Compared similar products with other materials, it has higher drilling speed, extends the service life 5 – 6 times, which not only saves time, improves the efficiency, but also reduces the frequency of changing buttons and manual labor.
With the increasing complexity of environmental conditions, drilling of drill performance requirements are also increasing, especially the button used for high-pressure drilling not only high hardness and wear resistance, but also need good toughness to prevent brittle fracture. The fine grain structure can effectively improve the performance of the alloy, studies have shown that ultrafine-grained and nano-structured carbide button binder phase content in the same case, when the WC grain size of less than 1μm, the hardness and strength can be greatly improved, and with the further reduction in WC grain size, the performance increase rate is more significant. Although nano grain is small in size, it has larger specific surface area, higher surface activity. So it has some excellent properties of nanostructures, such as lowering the sintering temperature required to increase the hardness, strength, wear resistance and impact toughness of mono button, life extension and so on, is one of the hotspots carbide materials currently.
Gradient carbide is a kind of multi-phase structured carbide that developed in the end of 1980s. The most outstanding characteristic is the gradient component and distribution. The preparation principle gradient structure of cemented carbide is obtained using low-carbon carbide alloy containing η phase by vacuum sintering, and treated to alter the distribution of Co binder phase of different parts of button distributed in different carburizing atmosphere Co content. As a result the organizational structure of tungsten carbide button shows gradient distribution of Co, the outer layer – Co-depleted layer, the intermediate layer – Co-rich layer, the inner layer – WC, Co, η three phases microstructure. The outer layer due to the high content of WC has good wear resistance; the intermediate layer has higher content of Co, so the toughness is better.

| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Trisulphide
- Details
- Category: Tungsten Information
- Published on Friday, 24 June 2016 16:43
- Written by Cristina
- Hits: 267

Tungsten trisulphide is a brown powder which becomes black when dried. When heated in absence of air it gives up sulphur and forms the disulphide WS2. It may be reduced to the metal by heating with lime in an electric furnace. When heated in the air it yields tungsten trioxide. It is slightly soluble in cold water, more readily in hot, forming a colloidal solution (see below). It is easily soluble in alkali hydroxides, carbonates, and sulphides, forming dark brown solutions which contain thiotungstates and colloidal tungsten trisulphide.
Colloidal Tungsten Trisulphide
It was observed by Berzelius that when the freshly precipitated trisulphide is washed with water, it dissolves to a slight extent, forming a yellow solution. If the precipitate is boiled with pure water it dissolves in considerable quantity, yielding a brownish-yellow solution. Winssinger obtained a dark brown solution of tungsten trisulphide by adding to a solution of sodium thiosulphate a little more dilute hydrochloric acid than was necessary to saturate the alkali. The solution could be passed through filter paper. On boiling, or on the addition of electrolytes, the tri-sulphide is precipitated.
A sulphochloride of tungsten, 3WS3.WCl6, has been obtained by heating the hexachloride with liquid hydrogen sulphide in a sealed tube for thirty-six hours at 60° to 65° C. It is a brown powder, insoluble in carbon disulphide, alcohol, or benzene; it is decomposed by water. If heated to redness in the air, it takes fire. It is readily oxidised by nitric acid or fused potash.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |





 sales@chinatungsten.com
sales@chinatungsten.com