Tungsten Copper Electrode Internal Influence Factors
- Details
- Category: Tungsten Information
- Published on Friday, 01 July 2016 14:59
- Written by xiaobin
- Hits: 245
Tungsten copper materials not only has high hardness, high strength, high melting point, high density, low coefficient of thermal expansion and excellent wear and corrosion resistance of W, but also has perfect plasticity and excellent thermal and electrical conductivity of Cu, so it has been widely used in electrical contact materials and electrode materials. But since there is a big difference in melting point and physical properties of them and completely immiscible, usually choose PM (Powder Metallurgy) as the manufacturing process, which also limit the range of applications of tungsten copper. At present, the major way for the preparation of tungsten copper composite material is W-Cu composite powder high energy ball milling, pressing, sintering, by analyzing the influence of grain size and composition of the powder itself and impurities brought sintering properties, in order to better control each key parameters to achieve high-performance tungsten copper electrode products.
In general, in the process of tungsten copper electrode manufacturing, in order to improve the compacts molding effect, we need to add some chemical agents, such as ethanol, stearic acid and so on. These additives may be directly volatilize or decompose in the subsequent sintering step and form pores between the particles. When the sintering temperature is rising, liquid Cu fluidity becomes poor and the gas can not escape completely and formed closed pores, which has a great effect on the density of tungsten copper electrode. In addition, these additives also likely to break down the formation of some C, H, O and other impurities, the smaller its diameter, has a strong expansion of capacity in the alloy. Thus it more likely to occur at a position higher energy grain boundary phase boundary segregation, etc., and even generate crisp phase, bringing the number of alloy intergranular fracture during fracturing along the increase, decrease overall performance. For the powder granularity, When the powder grain size small to some extent, powder sintering temperature decreased, surface area increased, sintering activity enhanced, which is beneficial for powder alloying. When the powder particle size to the nanometer range, the diffusion of powder has been greatly improved, densification process has been accelerated, and the final density of tungsten copper electrode has been improved at the same time.

| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Powder Applied in Manufacturing Bullet and Cartridge Case
- Details
- Category: Tungsten Information
- Published on Thursday, 30 June 2016 17:58
- Written by xinyi
- Hits: 266
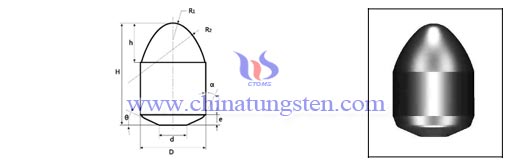
| Tungsten Powder Supplier: Chinatungsten Online tungsten-powder.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Rare Earth Affected Tungsten Electrode Welding Property
- Details
- Category: Tungsten Information
- Published on Thursday, 30 June 2016 16:07
- Written by yiping
- Hits: 308
To understand the impact of rare earth content on tungsten electrode welding property has significant for improving tungsten electrode welding performance. In the same production process, tungsten electrode with different rare earth content has different welding performance. As we all know, thorium tungsten electrodes have excellent welding performance, so as below we also compared rare earth tungsten electrode with thorium tungsten electrode.
Arc Starting Property
During experiment, the cathode is water cooled brass. Besides, the arc starting experiment starts form 30V and each interval increases by 1V no-load voltage. To carry out 30 times arc starting experiment for each voltage, the arc starting worked within 1 second is supposed to success and within 1 ~ 10 refers to arc starting lag behind, more than 10 seconds to arc which is failure. Learned form the experimental data shows that the rare earth tungsten electrode arc starting performance is better than thorium tungsten electrode. Rare earth tungsten electrode has low work function, so it has good arc performance. Doped with a variety of rare earth composite rare earth tungsten electrode arc starting property is superior to rare earth tungsten electrode doped with single rare earth oxide. Yttrium tungsten electrode high yttria content has better arc performance.
Anti-burning Performance
In experiment, the tungsten electrode links to the negative electrode, at 250A welding current arcing for 30 minutes. And then to use DP-100 optical analytical balance measured the electrode mass change before and after the experiment. From the experimental data shows that the mass of rare earth tungsten electrodes is higher than thorium tungsten electrode, namely, rare earth tungsten electrode has better anti-burning property than thorium tungsten electrode. Among different rare earth tungsten electrodes, yttrium tungsten electrode exhibits more excellent anti-burning property. When other conditions being equal, the lower work function on electrode surface is lower, the lower operating temperature, and anti-burning property is better. Rare earth tungsten electrode formed low work function active layer on the surface, so the anti-burning performance of the RE tungsten electrode is relatively good.

| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Rare Earth Affected Tungsten Electrode Arc Static Characteristic
- Details
- Category: Tungsten Information
- Published on Thursday, 30 June 2016 16:09
- Written by yiping
- Hits: 259
To learn tungsten electrode criticality arcing can understand the difficulty of arcing of electrode, which is one of the important property of the electrodes. However, currently, manufacturers usually using high-frequency for arcing, in general, as long as electrodes arc performance is similar to thorium tungsten electrode that arcing can be quite successful, but it hard to reflect rare earth tungsten electrode’s arcing advantage. Burning performance refers to the burned value of electrode under certain conditions, but it also differ greatly from the actual application operation, so only learned arc starting property and anti-burning property is not enough for complete testing electrode welding property. The welding arcing IU curve also known as arc static characteristic curve which can be more comprehensive performance tungsten electrodes’ welding property.
During experiment, the cathode is water-cooled brass, argon gas flow rate 6L / min, welding load voltage 70V, the arc length 3mm, and the electrode extraction air guide nozzle length is 6mm. After arcing quickly adjusted loop current to 40A, to respectively record the steady-state voltage value, when the current is at 40A, 60A, 80A, 100A, 120A, 140A, 160A, 180A and 200A and the arcing is stable, to obtain static characteristic curve.
From the arc static characteristic curve can be found rare earth tungsten electrode and thorium tungsten electrode has three distinct curves characters including droop characteristic area, flat characteristic area and rising characteristic area. Compared to the thorium tungsten electrode, all rare earth tungsten electrodes exhibit good static characteristics, showing excellent electron emission ability, and they can achieve particular arc current at a lower voltage. Tungsten electrode doped with small amount of rare earth oxide can significantly reduce the work function of the electrode surface and increase electron emission rate, so it has excellent welding property, mainly excited stable static characteristics, superior arc starting and anti-burning property. Doped with La2O3, CeO2 can improve electrode welding performance at small or medium current density. Doped with Y2O3 can improve electrode welding performance at high current density. Doped with a variety of rare earth oxides composite rare earth tungsten electrode has better welding performance than rare earth element tungsten electrodes doped with single rare earth oxide.

| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Iron, Cobalt and Tungsten Mixed OER Catalyst
- Details
- Category: Tungsten Information
- Published on Thursday, 30 June 2016 16:01
- Written by Cristina
- Hits: 274
Researchers at the University of Toronto in Canada and Stanford University in the US say that they have made the best oxygen-evolving reaction (OER) catalysts to date. The new catalysts, which are made from gelled multimetal oxy-hydroxides, have nanoporous structures and large specific surface areas containing lots of active sites for electrolytic reactions like the OER. The oxy-hydroxides can be prepared in a simple room-temperature process, which means that they might readily be scaled up to industrially relevant volumes.
The OER is one of the main processes occurring in metal-air batteries, solar water splitting and solar-powered fuel cells in general. It can be used to produce oxygen from water and as such could be a clean and renewable way to generate energy. Unfortunately, the reaction is rather slow and researchers are trying to find efficient catalyst materials to help speed it up. Although oxides based on ruthenium and iridium are good in this respect, these metals are expensive, which means they cannot realistically be used to make devices on the large scale.

Researchers say they have now discovered a new class of efficient catalyst for the OER based on the metals iron, cobalt and tungsten mixed together homogenously in an oxy-hydroxide framework that do have a nanoporous structure with large numbers of electrochemically active sites. The gelled multimetal structures can also be prepared via a simple room-temperature process, so they could easily be produced in large quantities.
The team began by dissolving precursors for all the metals in a polar organic solvent to form a homogenous solution. "By choosing suitable reagents, auxiliary agents and reaction conditions, we coordinated the hydrolysis of the different metal precursors, such that they were programmed to occur at similar rates to one another," explains Sargent. "This approach allowed different metal oxy-hydroxides to form simultaneously in an atomically homogenous distribution. Combining the metals in this way is challenging, since metals and metal oxides tend to phase-separate in crystalline oxides."

"Using state-of-the-art computational tools and powerful computers, we were able to understand, on the atomic scale, why and how the multimetal component oxide works," explains Vojvodic. "For example, an active site, where the OER takes place, containing cobalt, iron and, importantly, tungsten was found to be crucial."
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |





 sales@chinatungsten.com
sales@chinatungsten.com